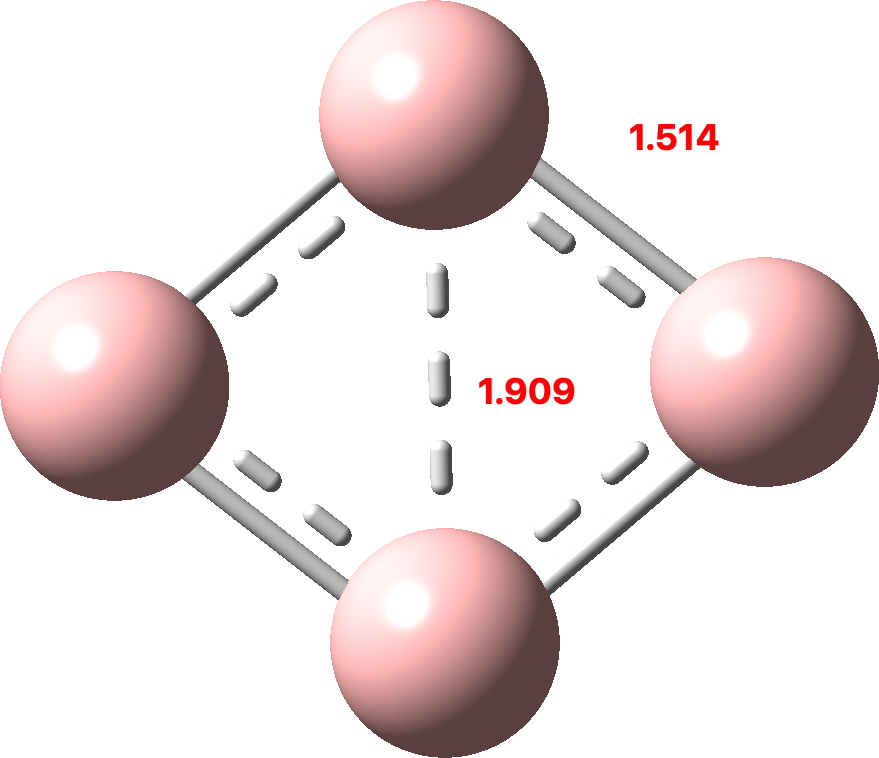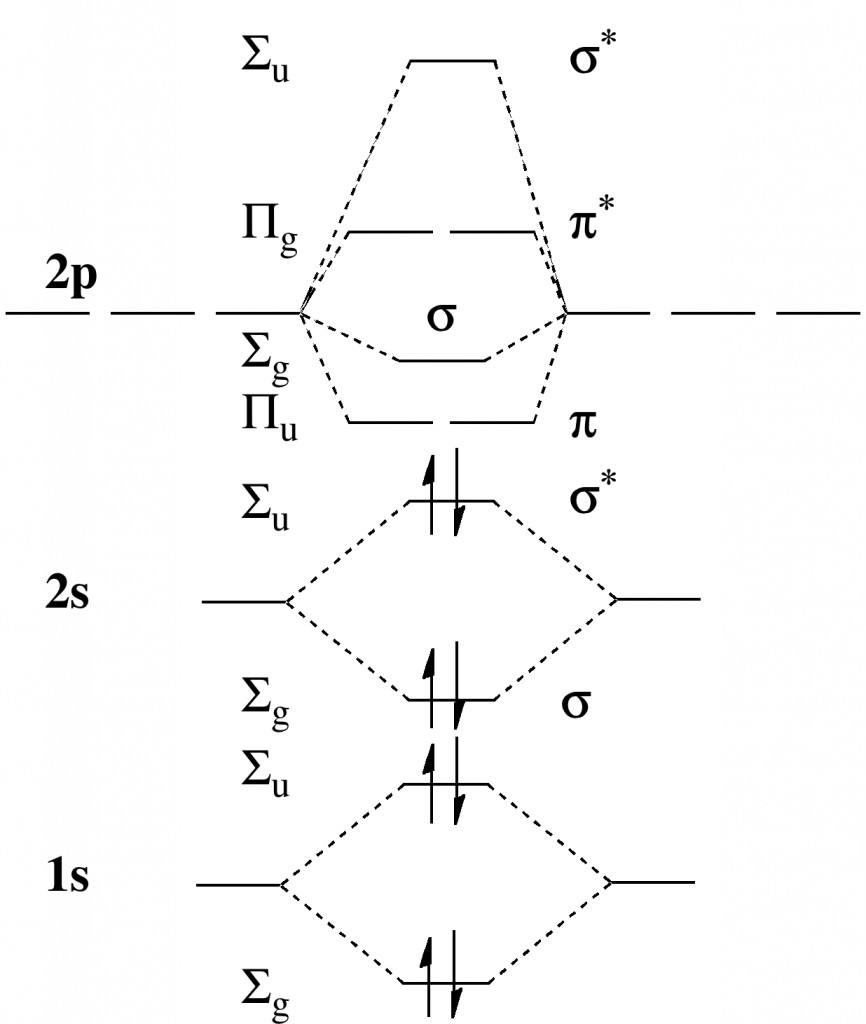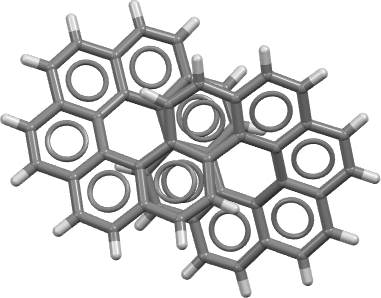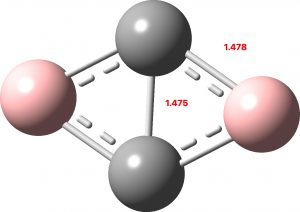
Normally, aromaticity is qualitatively assessed using an electron counting rule for cyclic conjugated rings. The best known is the Hückel 4n+2 rule (n=0,1, etc) for inferring diatropic aromatic ring currents in singlet-state π-conjugated cyclic molecules ‡ and a counter 4n rule which infers an antiaromatic paratropic ring current for the system.