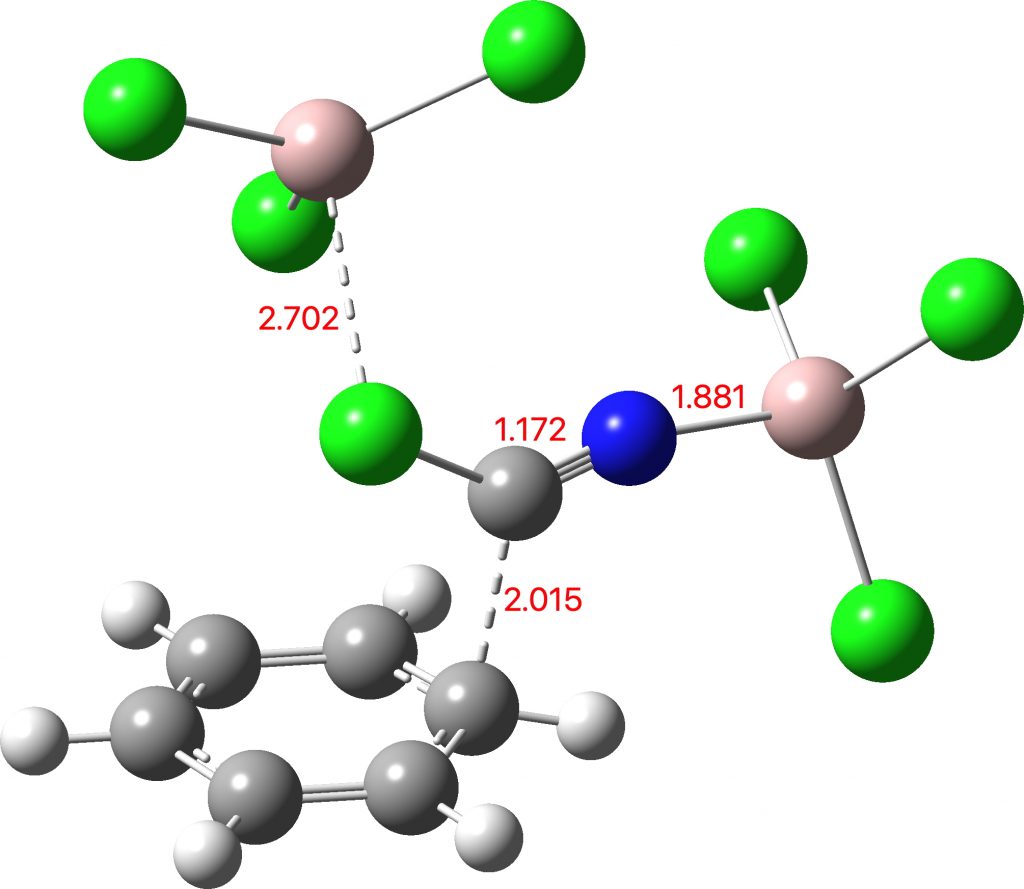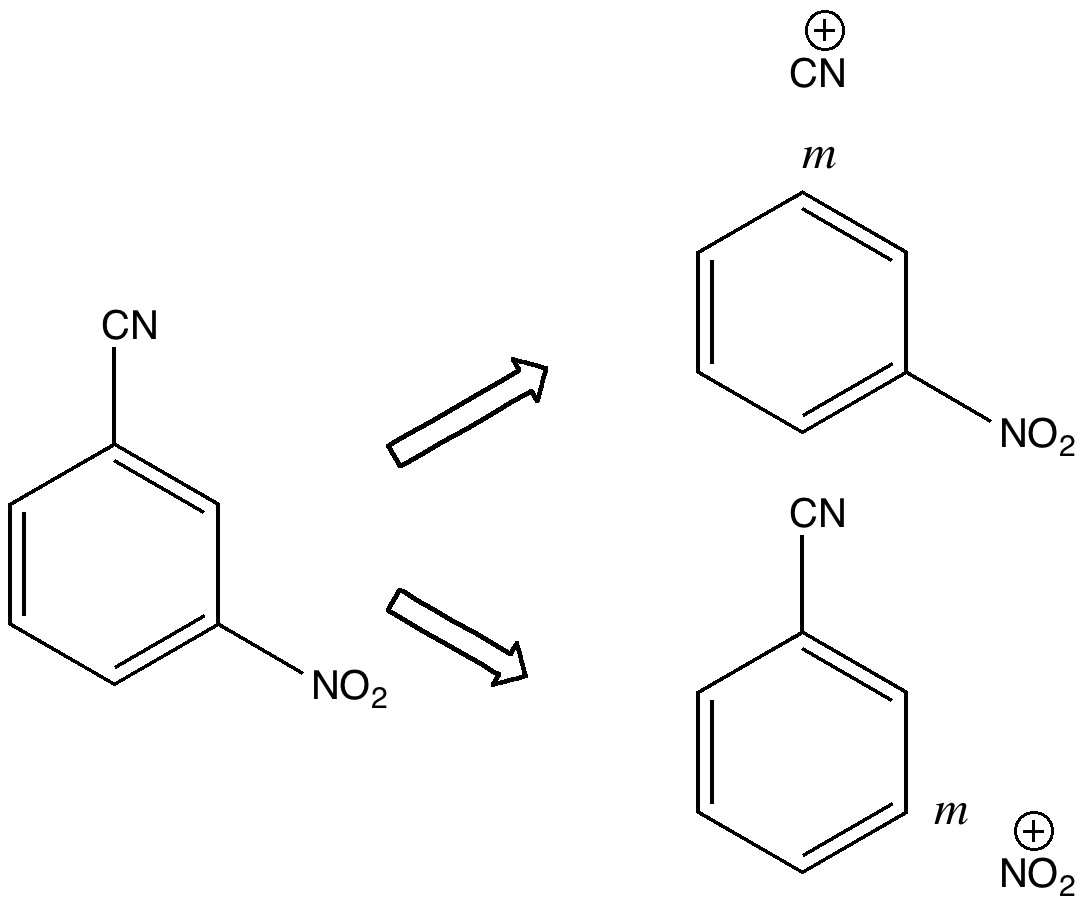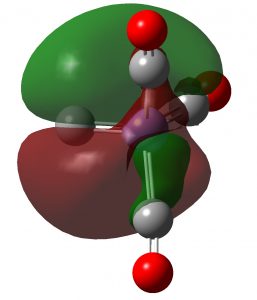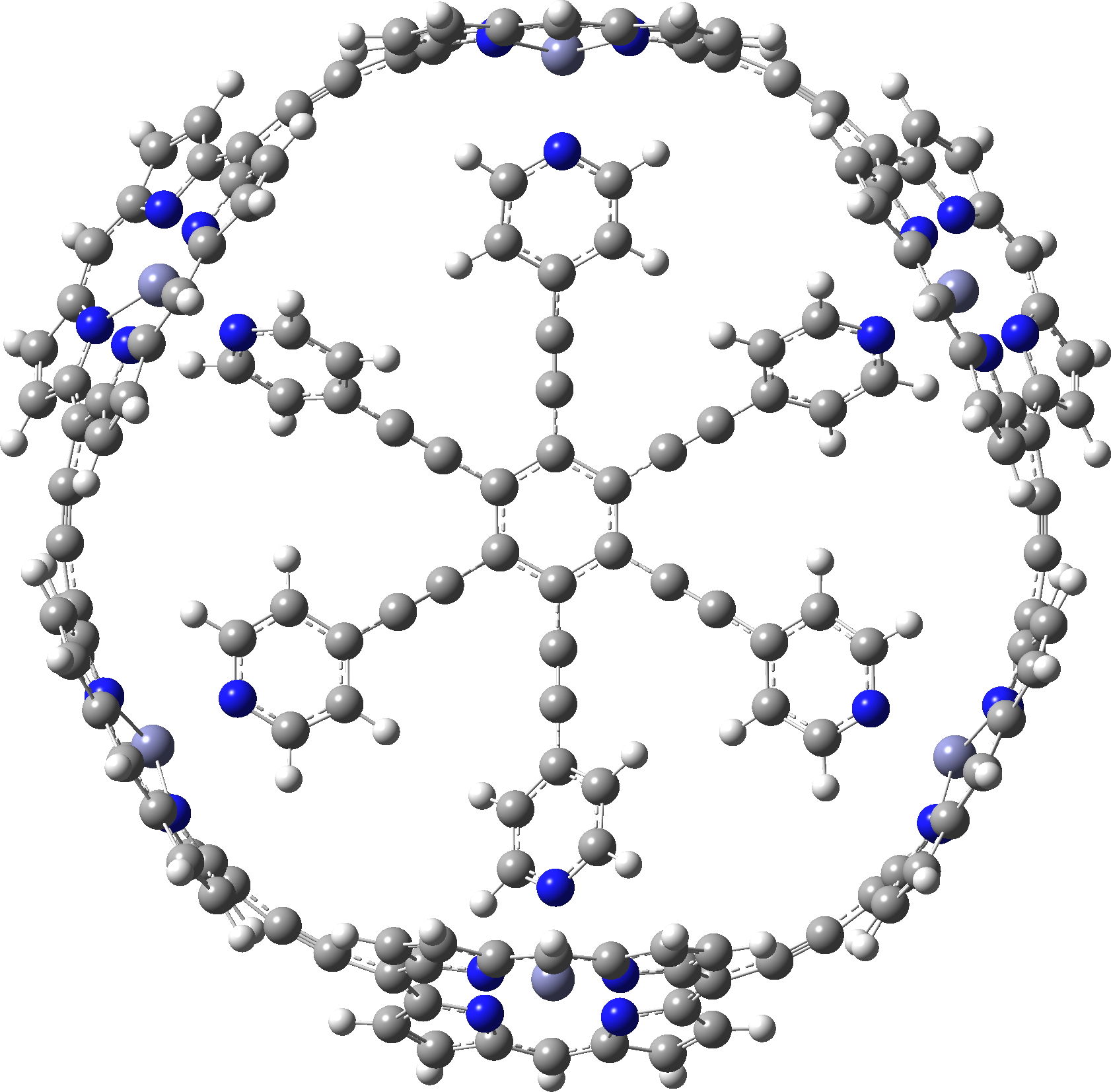
Here is another of the “large” molecules in the c&e news shortlist for molecule-of-the-year, 2020. This one is testing the Hückel 4n+2 rule out to a value never before seen (n = 40, or 162 π-electrons).[cite]10.1038/s41557-019-0398-3[/cite] The take-home message is that this rule seems to behave well in predicting global aromaticity even at this sort of scale!