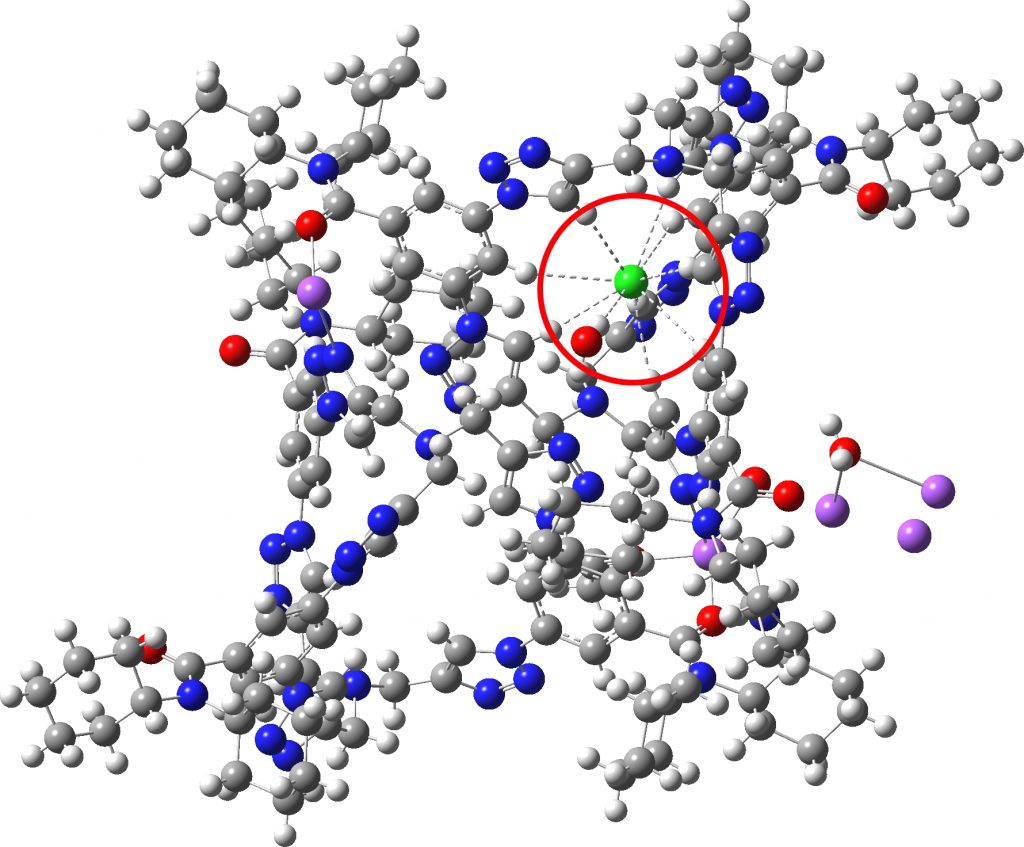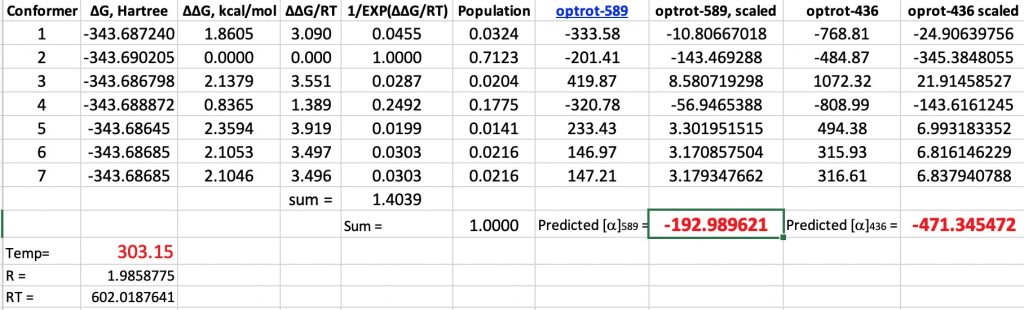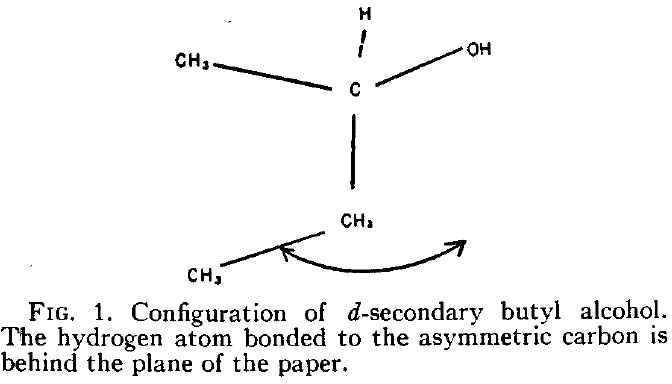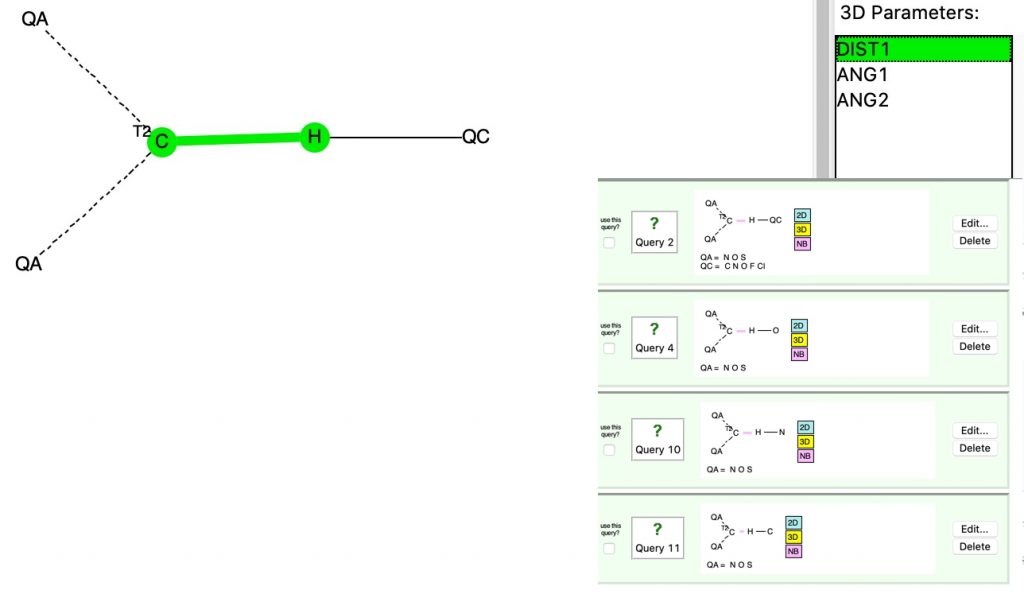
A hydrogen bond donor is considered as an electronegative element carrying a hydrogen that is accepted by an atom carrying a lone pair of electrons, as in X:…H-Y where X: is the acceptor and H-Y the donor. Wikipedia asserts that carbon can act as a donor, as we saw in the post on the incredible chloride cage, where six Cl**:**…H-C interactions trapped the chloride ion inside the cage.