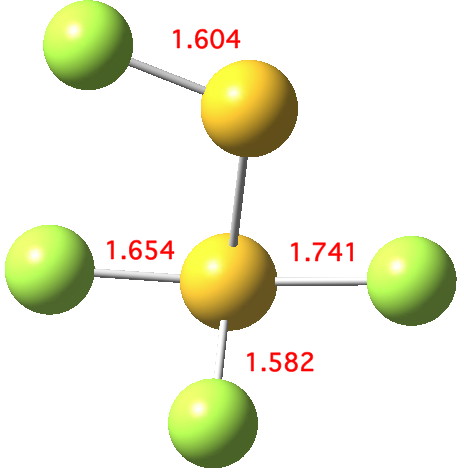
Andy Extance at the Chemistry World blog has picked up on a fascinating article[cite]10.1021/jz401578h[/cite] on the dimer of SF 2 . This molecule has three F atoms on one S, and only one on the other; FSSF 3 . But all four S-F bonds are of different length.