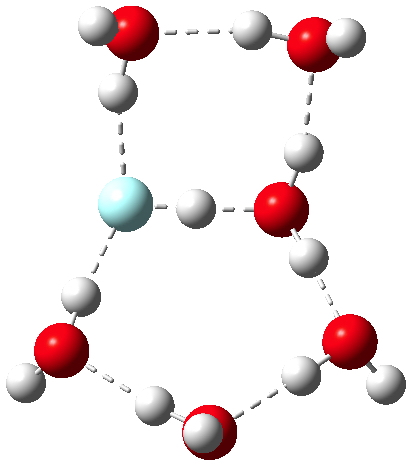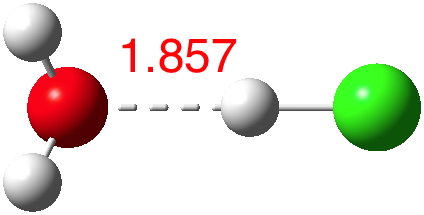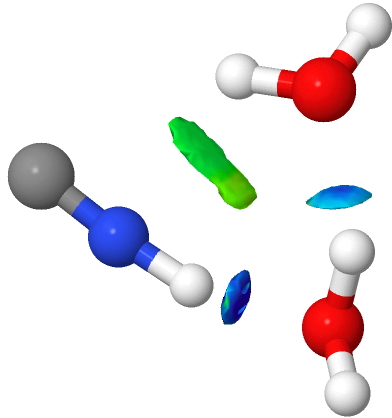
HCN is a weak acid (pKa +9.2, weaker than e.g. HF), although it does have an isomer, isocyanic acid or HNC (pka < +9.2 ?) which is simultaneously stronger and less stable. I conclude my halide acid series by investigating how many water molecules (in gas phase clusters) are required for ionisation of this “ pseudo-halogen ” acid.