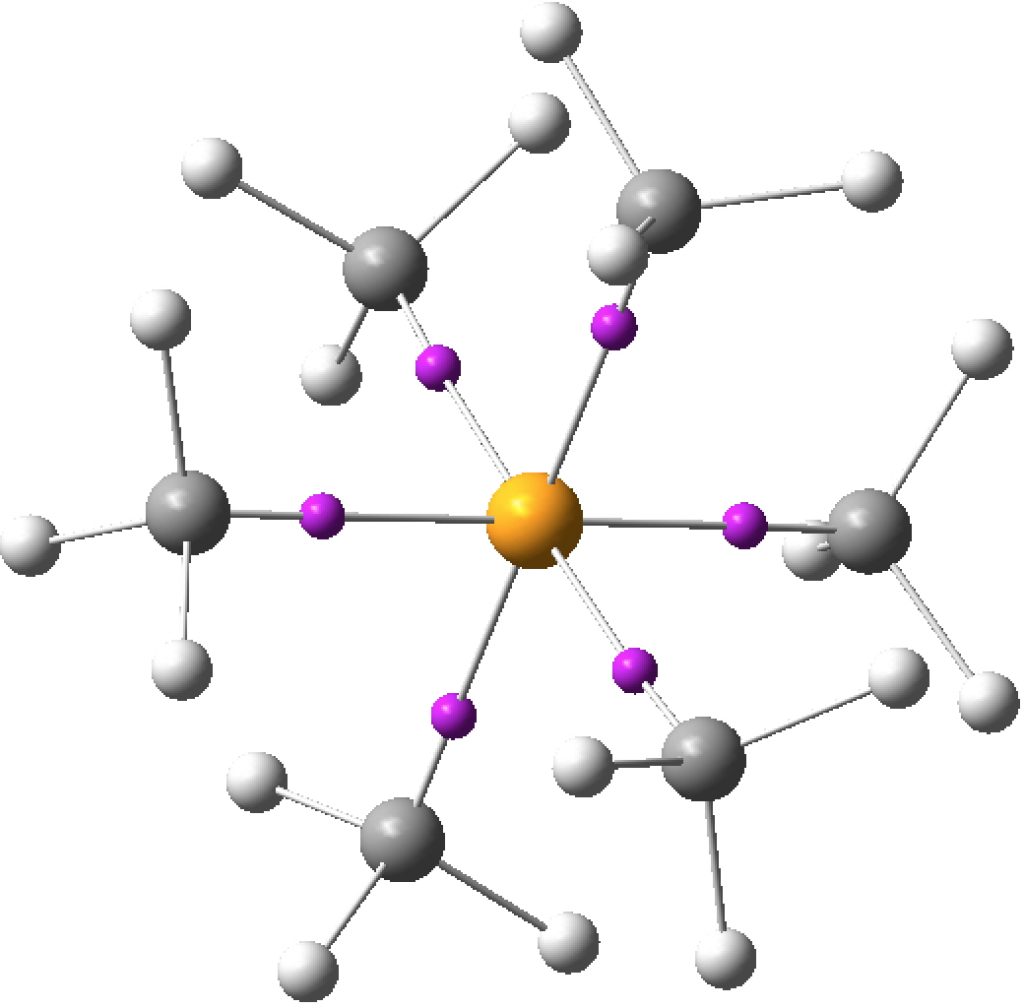
I started this story by looking at octet expansion and hypervalence in non-polar hypercoordinate species such as S(-CH 3 ) 6 , then moved on to S(=CH 2 ) 3 . Finally now its the turn of S(≡CH) 2 . ‡ As the triple bonds imply, this seems to represent twelve shared valence electrons surround the sulfur, six from S itself and three from each carbon.
Postagens de Rogue Scholar
Previously: “Non-polar” species such as SeMe6, SMe6, ClMe3, ClMe5 all revealed interesting properties for the Se-C, S-C or Cl-C “single” bonds. The latter two examples in particular hinted at internal structures for these single bonds, as manifested by two ELF basins for some of the bonds.
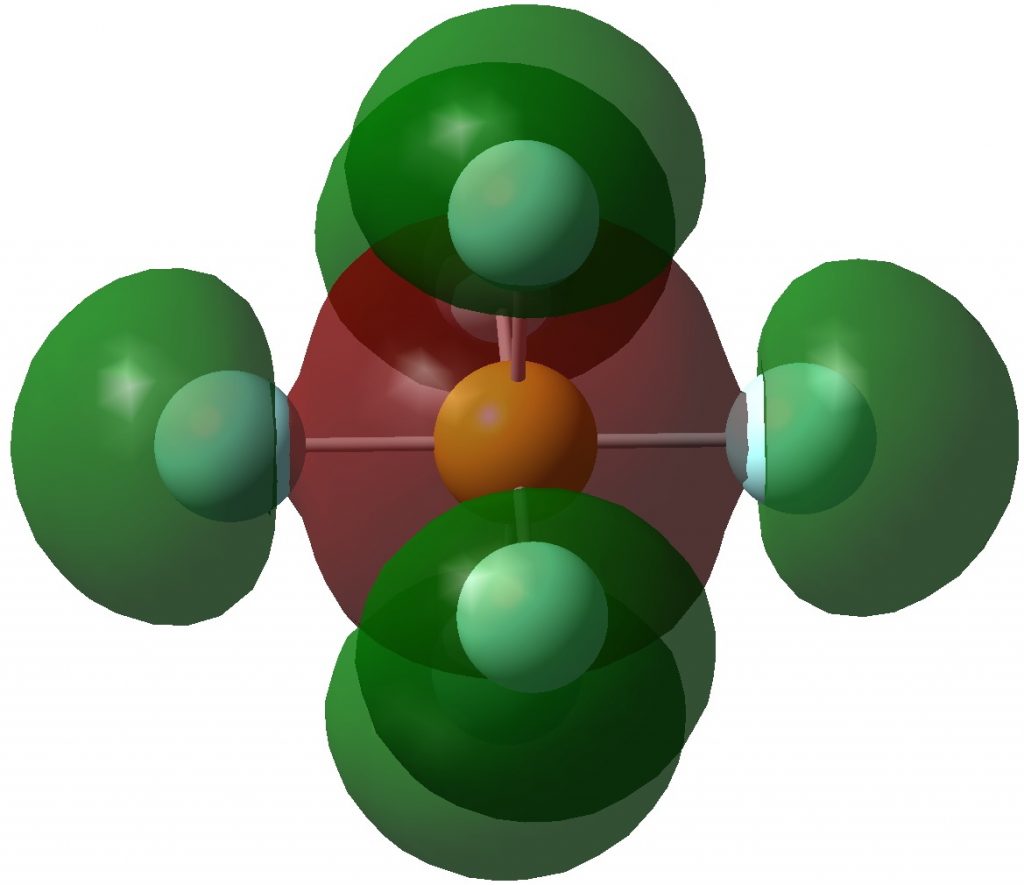
Following on from discussing octet expansion in species such as SeMe 6 , ClMe 3 and ClMe 5 , I felt impelled to return to SF 6 , often used as an icon for hypervalence.
A few years back, I took a look at the valence-shell electron pair repulsion approach to the geometry of chlorine trifluoride, ClF 3 using so-called ELF basins to locate centroids for both the covalent F-Cl bond electrons and the chlorine lone-pair electrons.
One thread that runs through this blog is that of hypervalency. It was therefore nice to come across a recent review of the concept[cite]10.1039/c5sc02076j[/cite] which revisits the topic, and where a helpful summary is given of the evolving meanings over time of the term hypervalent.
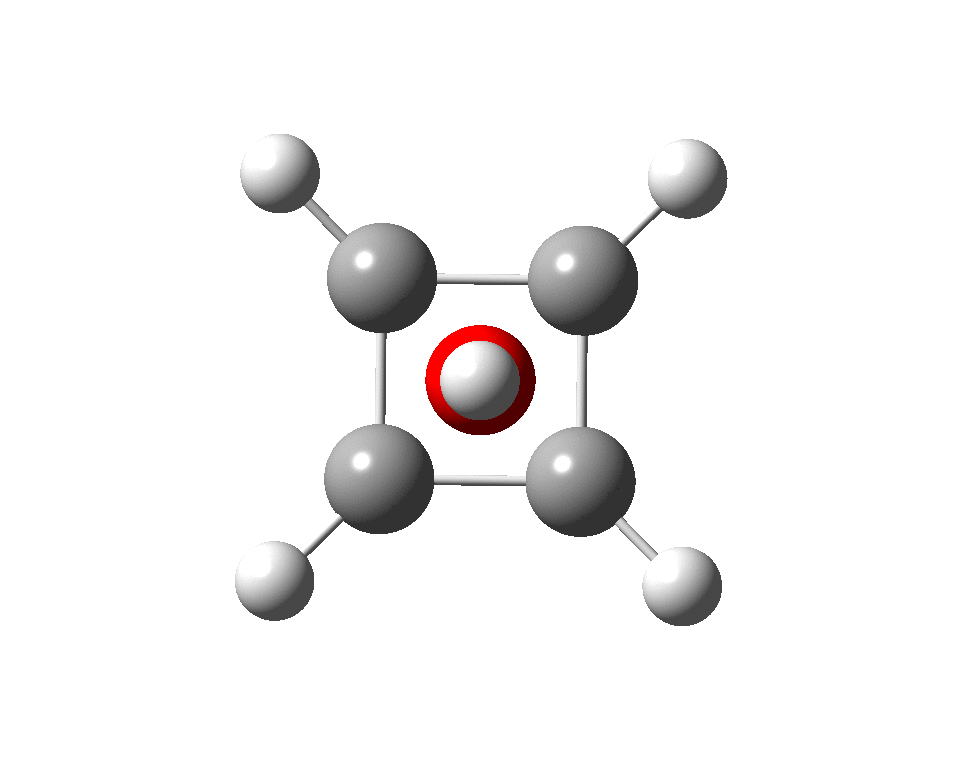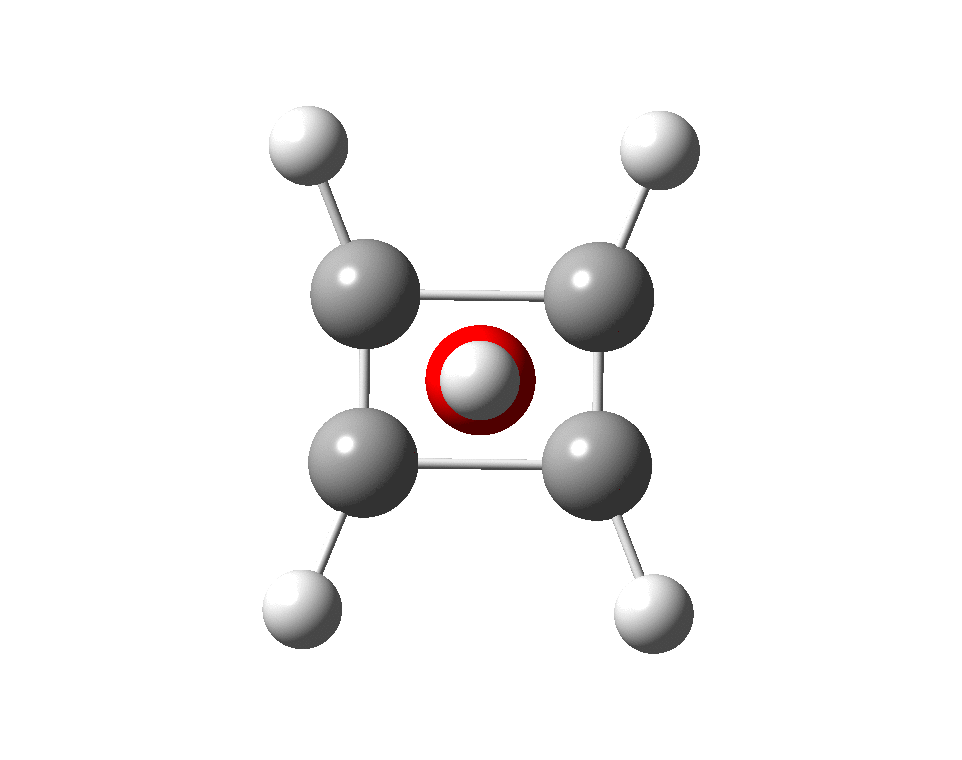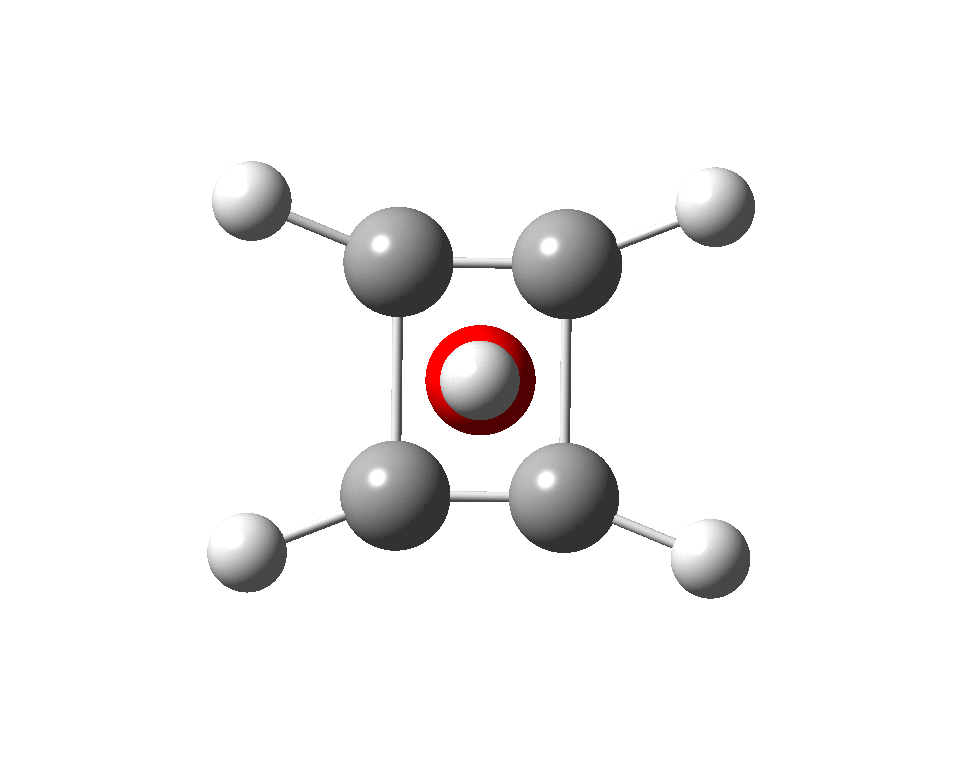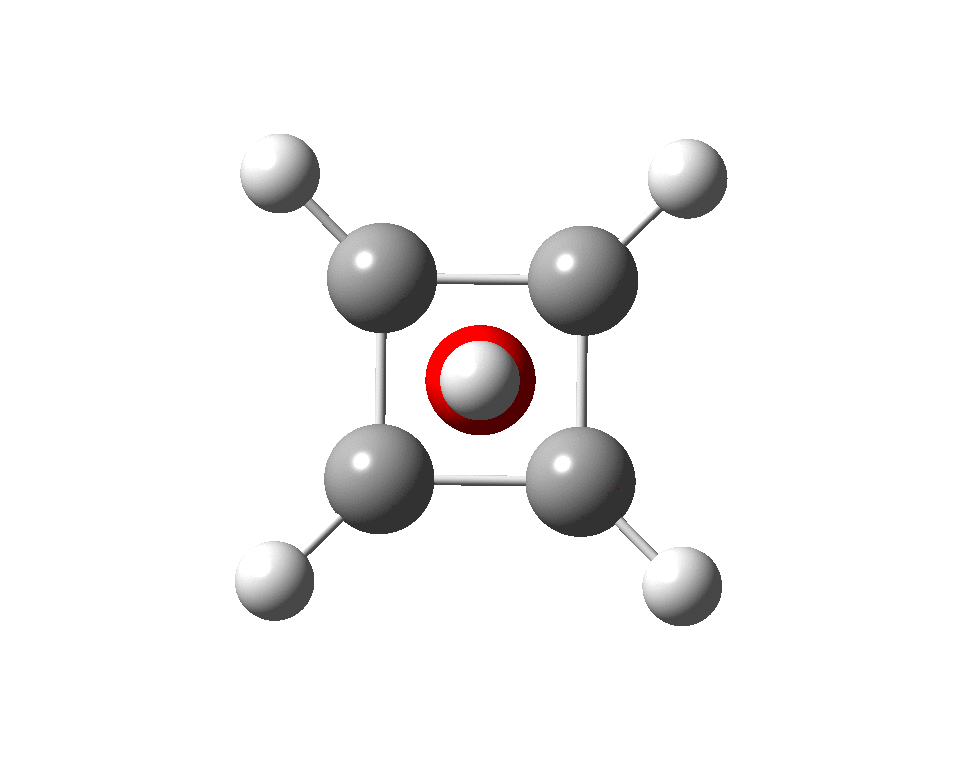
The previous post demonstrated the simple iso-electronic progression from six-coordinate carbon to five coordinate nitrogen. Here, a further progression to oxygen is investigated computationally. The systems are formally constructed from a cyclobutadienyl di-anion and firstly the HO 5+ cation, giving a tri-cationic complex. There are no examples of the resulting motif in the Cambridge structure database.
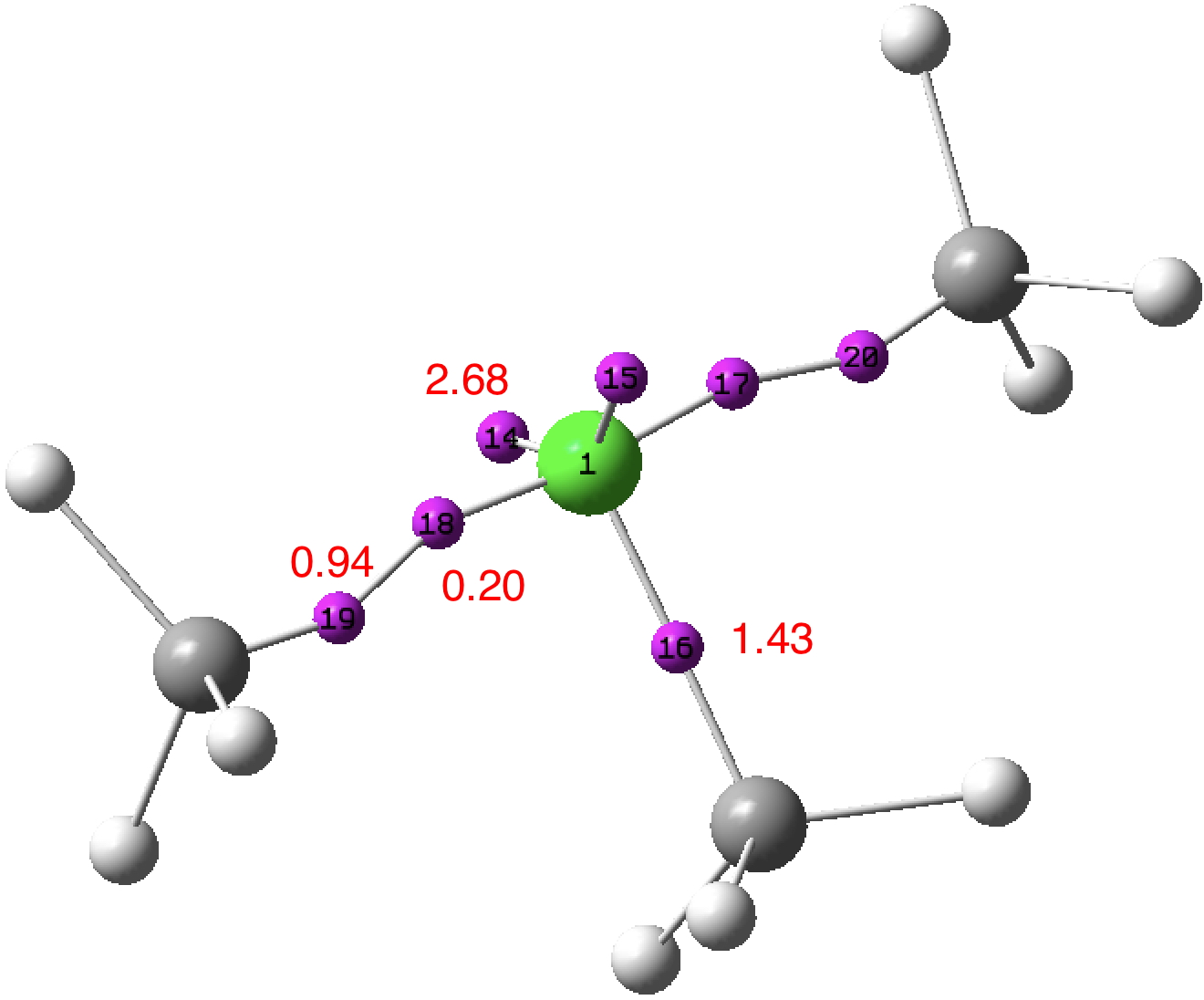
A few years back I followed a train of thought here which ended with hexacoordinate carbon, then a hypothesis rather than a demonstrated reality. That reality was recently confirmed via a crystal structure, DOI:10.5517/CCDC.CSD.CC1M71QM[cite]10.1002/anie.201608795[/cite]. Here is a similar proposal for penta-coordinate nitrogen. First, a search of the CSD (Cambridge structure database) for such nitrogen.

Hypervalency is defined as a molecule that contains one or more main group elements formally bearing more than eight electrons in their valence shell. One example of a molecule so characterised was CLi 6 [cite]10.1038/355432a0[/cite] where the description "“ carbon can expand its octet of electrons to form this relatively stable molecule “ was used.
The post on applying VSEPR ("valence shell electron pair repulsion") theory to the geometry of ClF 3 has proved perennially popular. So here is a follow-up on another little molecue, F 3 SN. As the name implies, it is often represented with an S≡N bond. Here I take a look at the conventional analysis. This is as follows: Six valence electrons on the central S atom.
Science is rarely about a totally new observation or rationalisation, it is much more about making connections between known facts, and perhaps using these connections to extrapolate to new areas (building on the shoulders of giants, etc). So here I chart one example of such connectivity over a period of six years.