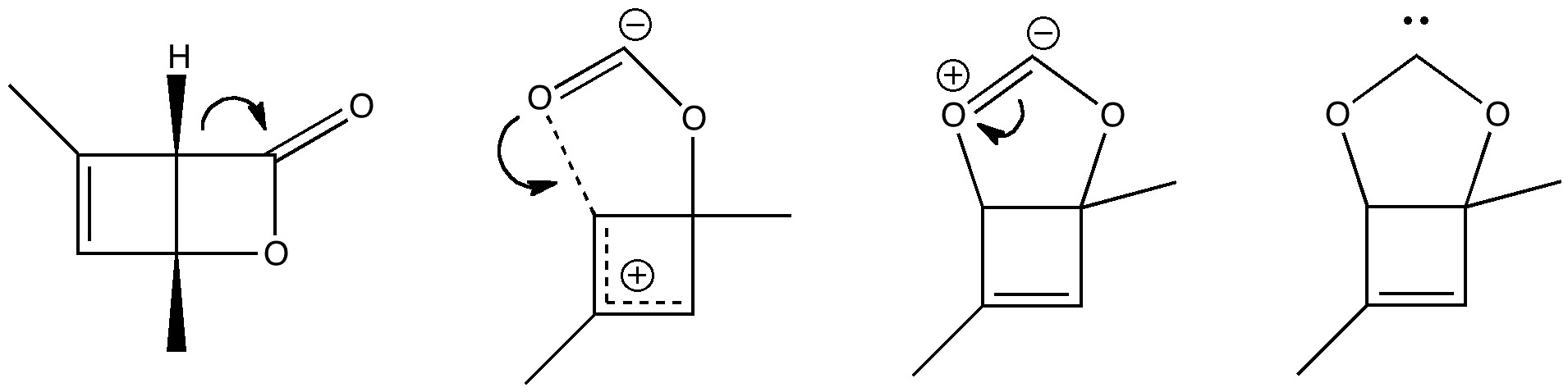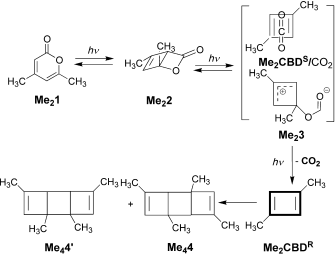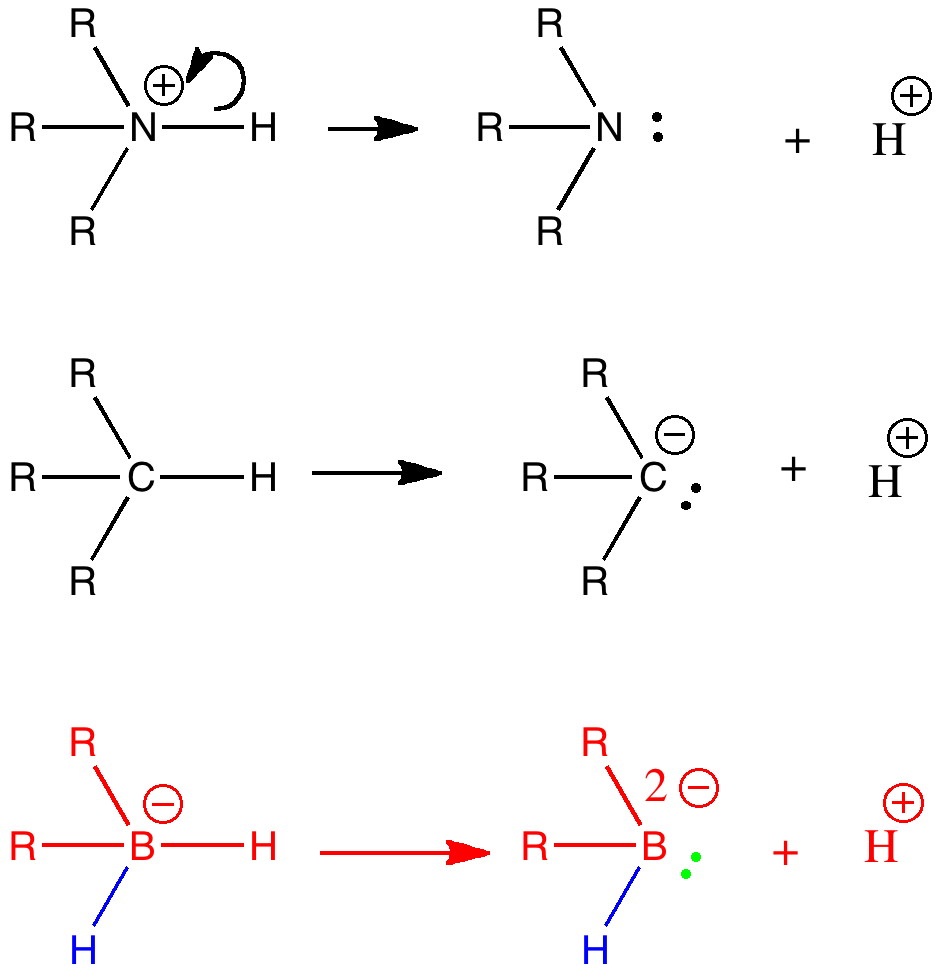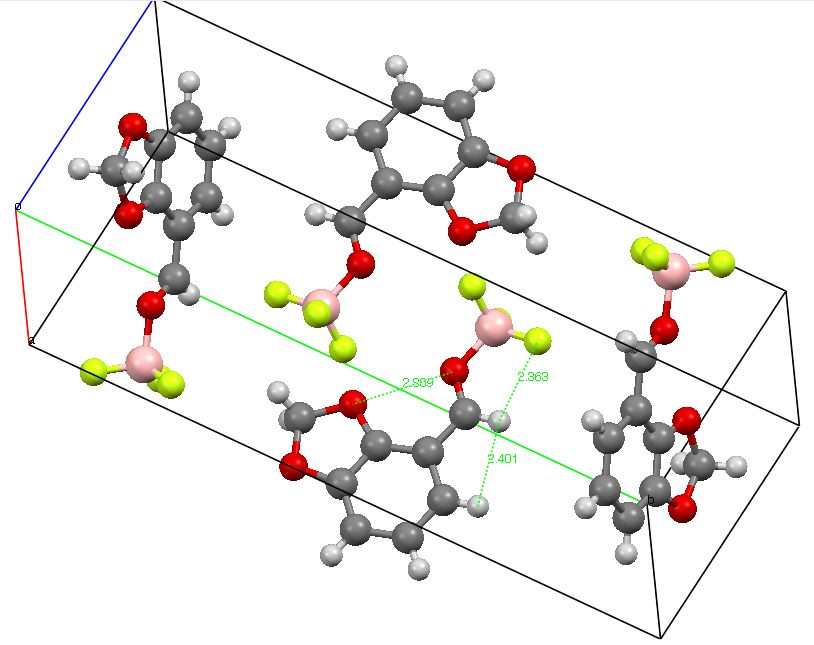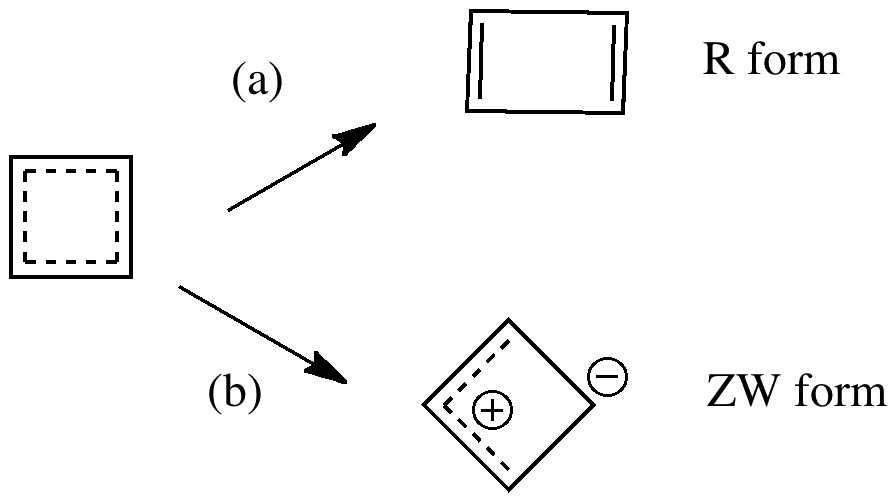
Organic chemistry has some no-go areas, where few molecules dare venture. One of them is described by a concept known as anti-aromaticity. Whereas aromatic molecules are favoured species, their anti-equivalent is avoided. I previously illustrated this (Hückel rule) with cyclopropenium anion.