If you visit this blog you will see a scientific discourse in action.

If you visit this blog you will see a scientific discourse in action.
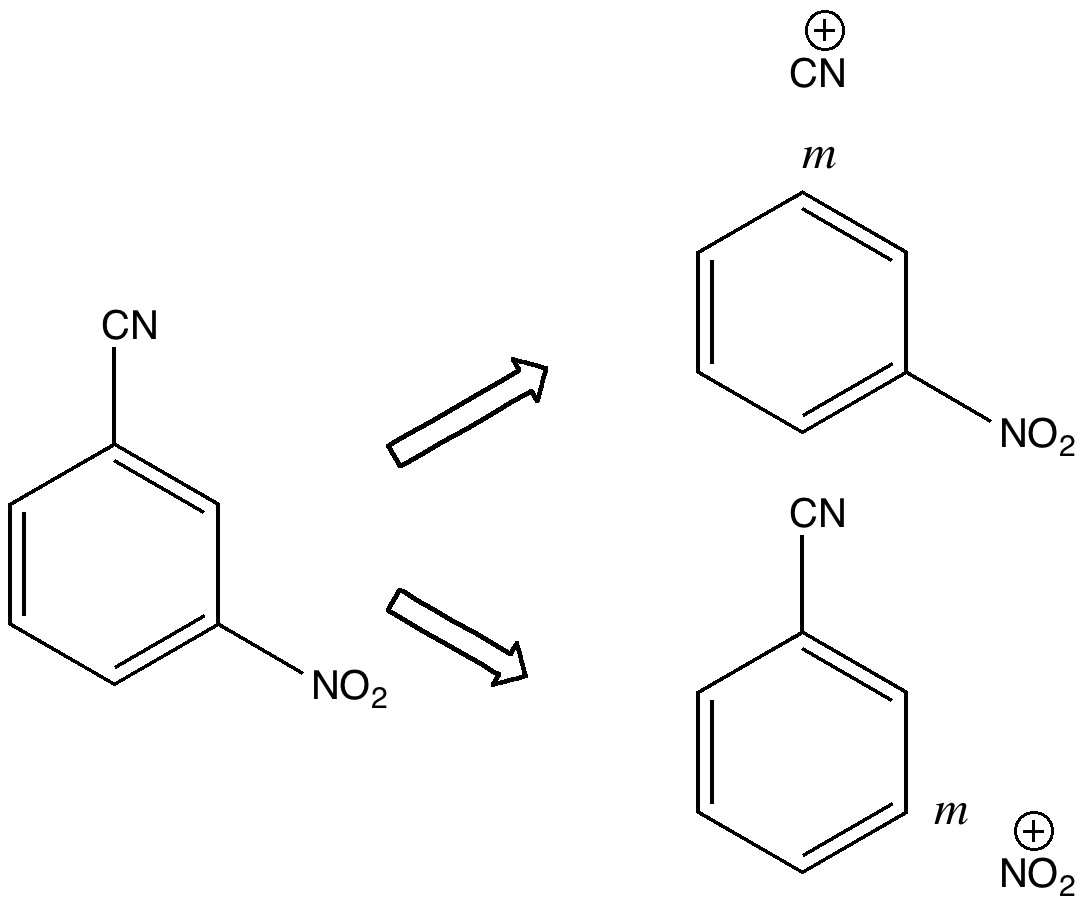
Do you fancy a story going from simplicity to complexity, if not absurdity, in three easy steps? Read on! The following problem appears in one of our (past) examination questions in introductory organic chemistry. From relatively mundane beginnings, one can rapidly find oneself in very unexpected territory. How would one make 3-nitrobenzonitrile?
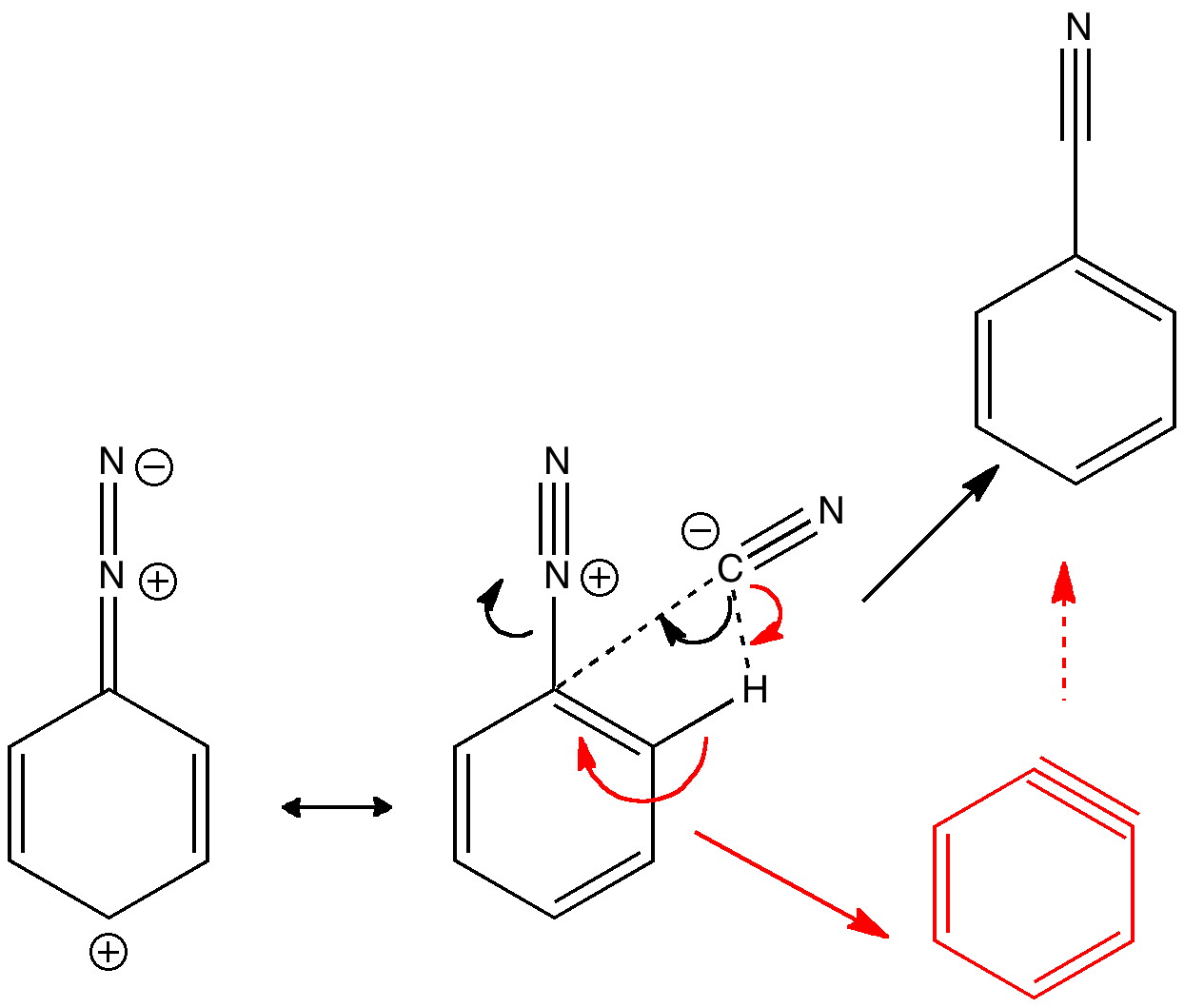
Janus was the mythological Roman god depicted as having two heads facing opposite directions, looking simultaneously into the past and the future. Some of the most ancient ( i.e. 19th century) known reactions can be considered part of a chemical mythology; perhaps it is time for a Janus-like look into their future. Reaction of the diazonium cation with cyanide.
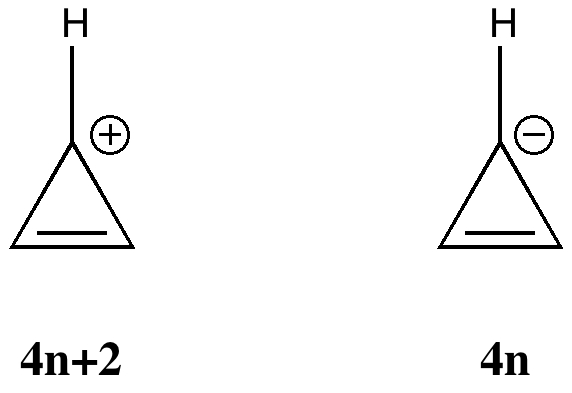
More inspiration from tutorials. In a lecture on organic aromaticity, the 4n+2/4n Hückel rule was introduced (in fact, neither rule appears to have actually been coined in this form by Hückel himself!). The simplest examples are respectively the cyclopropenyl cation and anion. The former has 2 π-electrons exhibiting cyclic delocalisation, and the 4n+2 (n=0) rule predicts aromaticity.

My university tutorial yesterday covered selective reductions of functional groups in organic chemistry. My thoughts on that topic have now morphed into something rather different. Scientific research has a habit of having this sort of thing happen.
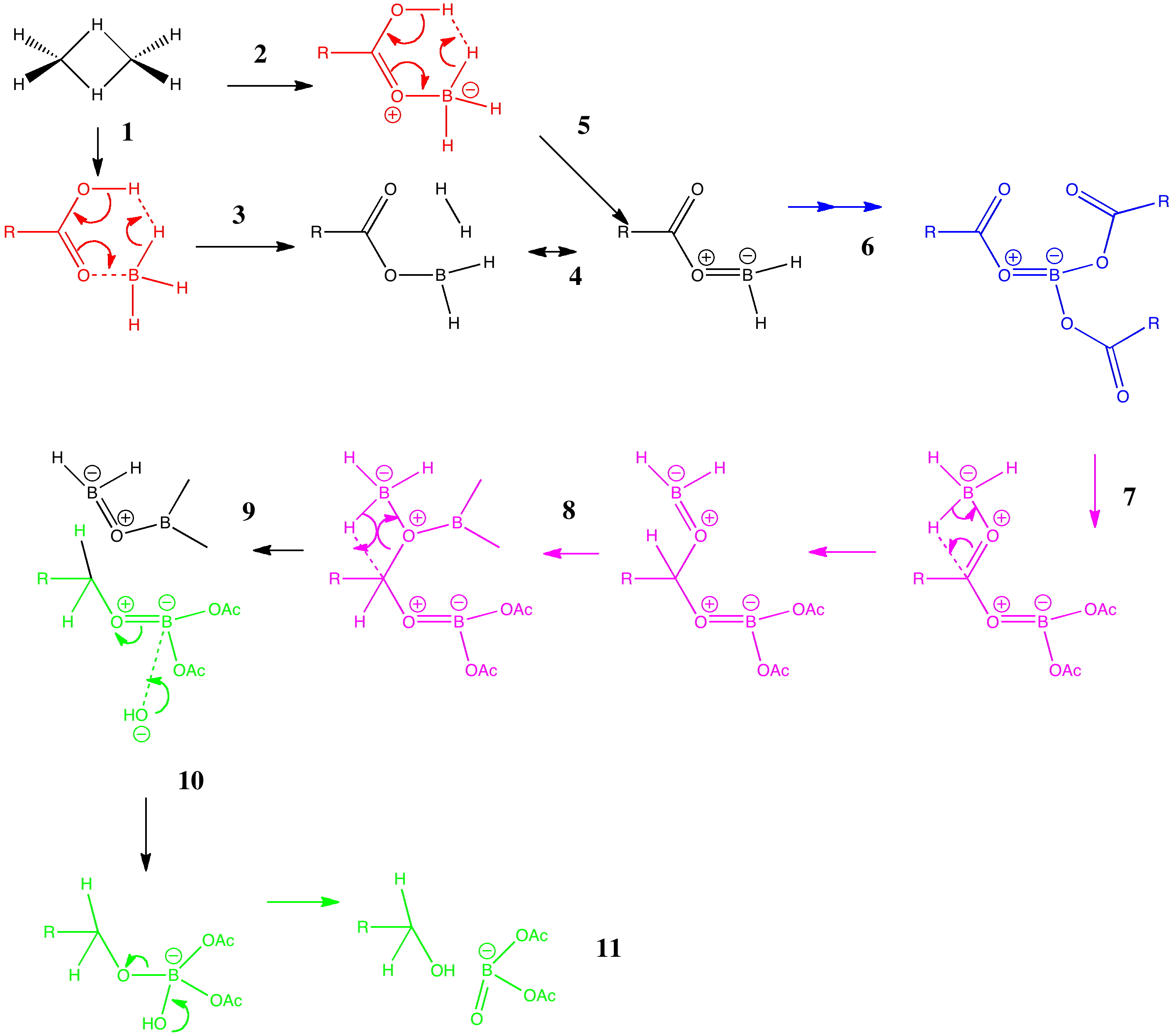
Arrow pushing (why never pulling?) is a technique learnt by all students of organic chemistry (inorganic chemistry seems exempt!). The rules are easily learnt (supposedly) and it can be used across a broad spectrum of mechanism. But, as one both becomes more experienced, and in time teaches the techniques oneself as a tutor, its subtle and nuanced character starts to dawn.
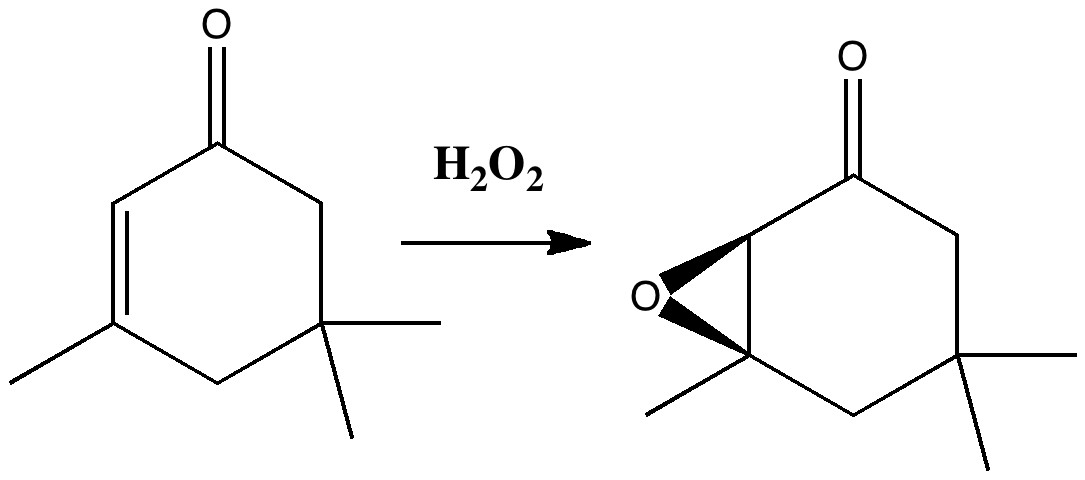
Our understanding of science mostly advances in small incremental and nuanced steps (which can nevertheless be controversial) but sometimes the steps can be much larger jumps into the unknown, and hence potentially more controversial as well. More accurately, it might be e.g. relatively unexplored territory for say a chemist, but more familiar stomping ground for say a physicist.

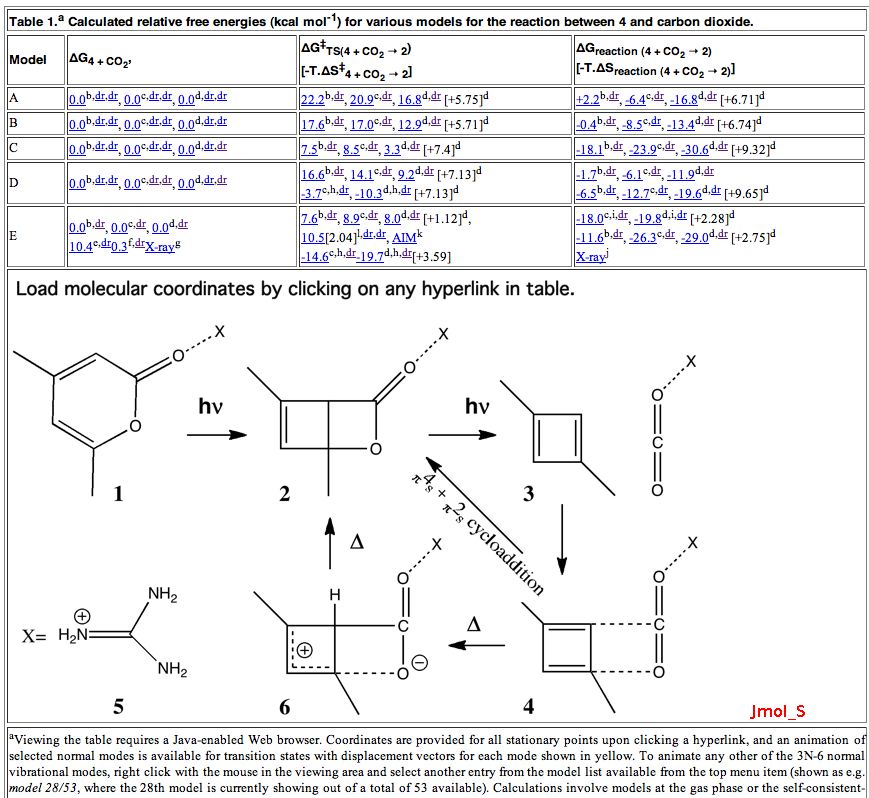
On 8th August this year, I posted on a fascinating article that had just appeared in Science[cite]10.1126/science.1188002[/cite] in which the crystal structure was reported of two small molecules, 1,3-dimethyl cyclobutadiene and carbon dioxide, entrapped together inside a calixarene cavity.
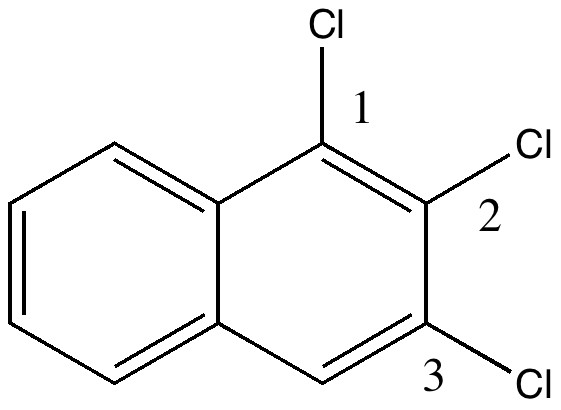
In 1890, chemists had to work hard to find out what the structures of their molecules were, given they had no access to the plethora of modern techniques we are used to in 2010. For example, how could they be sure what the structure of naphthalene was? Well, two such chemists, William Henry Armstrong (1847-1937) and his student William Palmer Wynne (1861-1950;
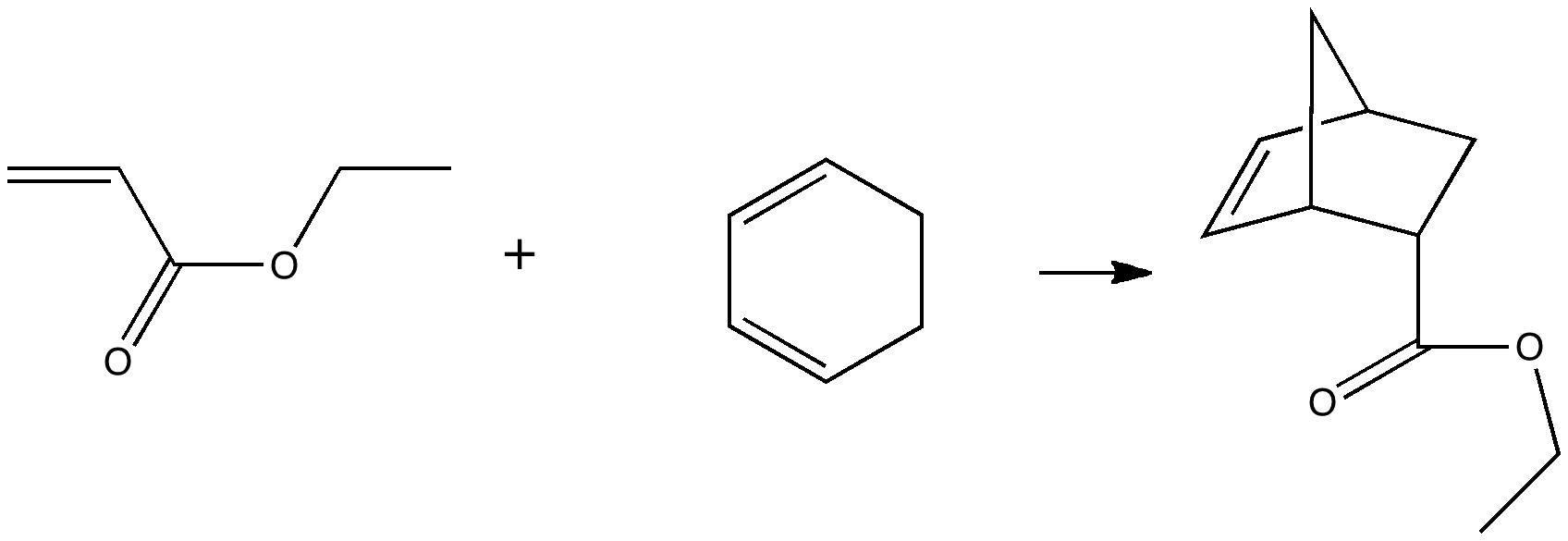
Reactions in cavities can adopt quite different characteristics from those in solvents. Thus first example of the catalysis of the Diels-Alder reaction inside an organic scaffold was reported by Endo, Koike, Sawaki, Hayashida, Masuda, and Aoyama[cite]10.1021/ja964198s[/cite], where the reaction shown below is speeded up very greatly in the presence of a crystalline lattice of the anthracene derivative shown below. A Diels-Alder reaction.