There are many treasures in Woodward and Hoffmann’s (WH) classic monograph. One such is acetolysis of the endo chloride (green), which is much much faster than that of the exo isomer (red). The explanation given in their article (p 805) confines itself to succinctly stating that only loss of the endo halogen can be concerted with a required disrotatory ring opening of the cyclopropane.
Postagens de Rogue Scholar
Thus famously wrote Woodward and Hoffmann (WH) in their classic monograph about the conservation of orbital symmetry in pericyclic reactions.
My previous three posts set out my take on three principle categories of pericyclic reaction. Here I tell a prequel to the understanding of these reactions. In 1965, Woodward and Hoffmann[cite]10.1021/ja01080a054[/cite] in their theoretical analysis (submitted Nov 30, 1964) for which the Nobel prize (to Hoffmann only of the pair, Woodward having died) was later awarded.
Another common type of pericyclic reaction is the migration of hydrogen or carbon along a conjugated chain, as in the [1,3] migration of a carbon as shown below. As before, I explore the stereochemistry of the thermal and photochemical reactions.
The π2 + π2 cyclodimerisation of cis-butene is the simplest cycloaddition reaction with stereochemical implications. I here give it the same treatment as I did previously for electrocyclic pericyclic reactions.
The epoxidation of an alkene to give an oxirane is taught in introductory organic chemistry. Formulating an analogous mechanism for such reaction of an alkyne sounds straightforward, but one gradually realises that it requires raiding knowledge from several other areas of (perhaps slightly more advanced) chemistry to achieve a joined up approach to the problem.
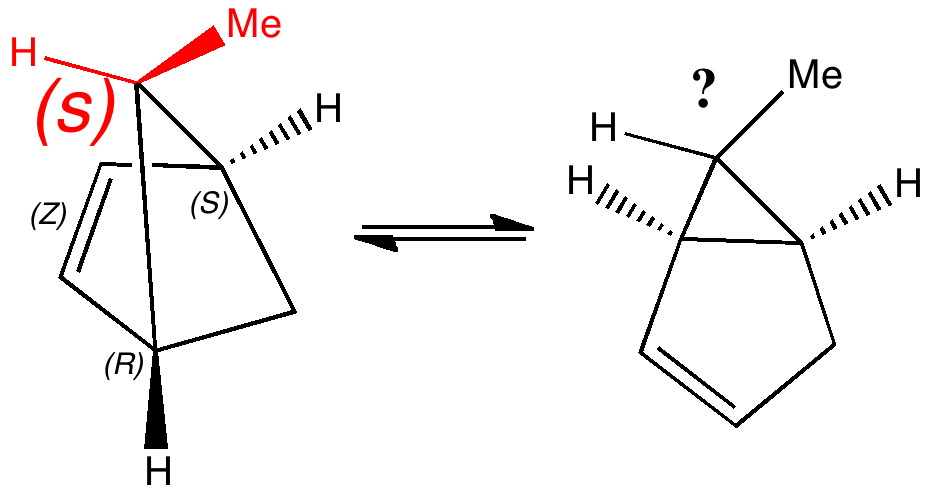
Most representational chemistry generated on a computer requires the viewer to achieve a remarkably subtle transformation in their mind from two to three dimensions (we are not quite yet in the era of the 3D iPad!). The Cahn-Ingold-Prelog convention was a masterwork (which won the Nobel prize). It is shown in action for the molecule on the left below.
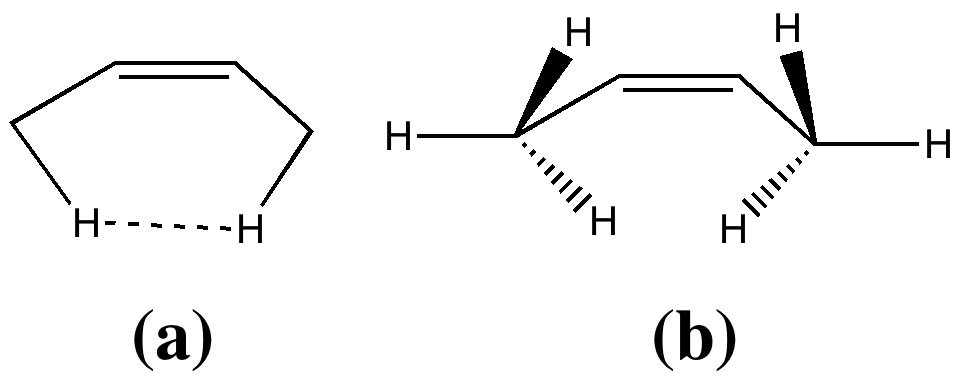
In two previous posts, I have looked at why cis -butene adopts conformation (a) rather than (b). I suggested it boiled down to electronic interactions between the methyl groups and the central alkene resulting in the formation of a H…H “ topological ” bond, rather than attraction between the H…H region to form a weak chemical “ bond ”. Here I take a look at what happens when that central C=C bond is gradually removed.
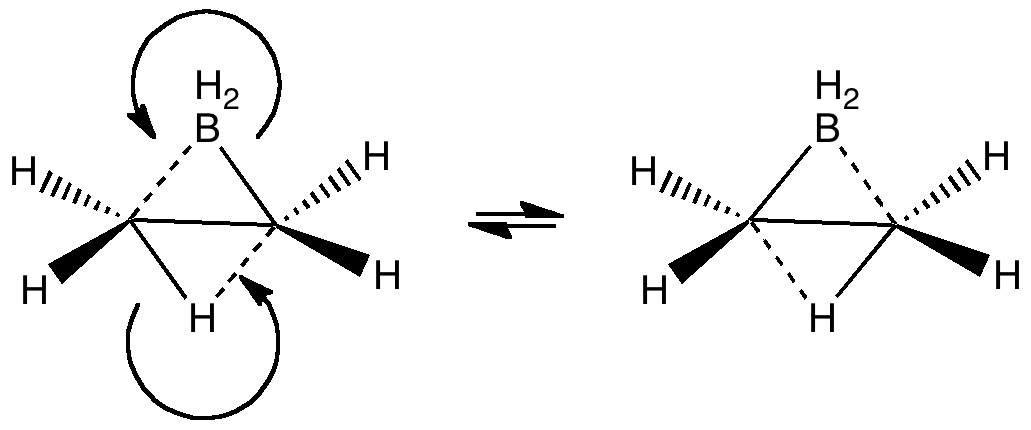
The last two posts have played a game of find the electrons. We saw how the dyotropic rearrangement of ethane borrowed electrons from the C-C bond, and how 1,2,dibromoethane went ionic on us. How about this mixed system, in which a hydrogen and a BH 2 swap their positions? Dyotropic rearrangement involving boron and hydrogen. It is yet again different.

In the previous post, I discussed what we could learn from ethane by forcing it into a pericyclic dyotropic rearrangement. We saw how it voraciously scavenged two electrons from the C-C bond to achieve this. What if we give it more electrons? Thus 1,2-dibromoethane undergoing the same reaction. Dyotropic rearrangement of 1,2-dibromoethane.