In a welcome move, one of the American chemical society journals has published an encouragement to submit what is called FAIR data to the journal.[cite]10.1021/acs.orglett.0c00383[/cite]. A reminder that FAIR data is data that can be Found (F), Accessed (A), Interoperated(I) and Re-used( R). I thought I might try to explore this new tool here. You start at the ACS Research Data Center
Postagens de Rogue Scholar
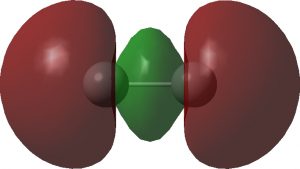
I noted in an earlier blog, a potential (if difficult) experimental test of the properties of the singlet state of dicarbon, C 2 . Now, just a few days ago, a ChemRxiv article has been published suggesting another (probably much more realistic) test.[cite]10.26434/chemrxiv.11446224.v1[/cite] This looks at the so-called 7 Σ open shell state of the molecule where three electrons from one σ and two π orbitals are excited
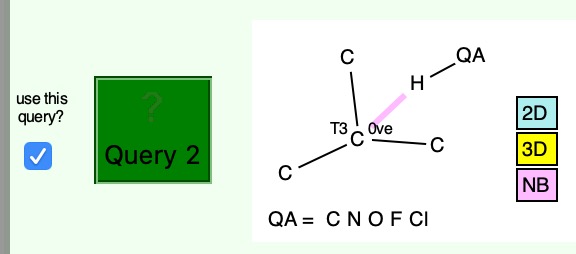
Having shown that carbon as a carbene centre, C**:** can act as a hydrogen bond acceptor, as seen from a search of crystal structures, I began to wonder if there is any chance that carbon as a radical centre, C• could do so as well. Definitely a subversive thought, since radical centres are supposed to abstract hydrogens rather than to hydrogen bond to them.
In the previous post, I showed that carbon can act as a hydrogen bond acceptor (of a proton) to form strong hydrogen bond complexes. Which brings me to a conceptual connection: can singlet dicarbon form such a hydrogen bond? Dicarbon can be variously represented as above. The first form shows it as a bis-carbene, with an unbonded lone pair of electrons at each end of a carbon double bond.
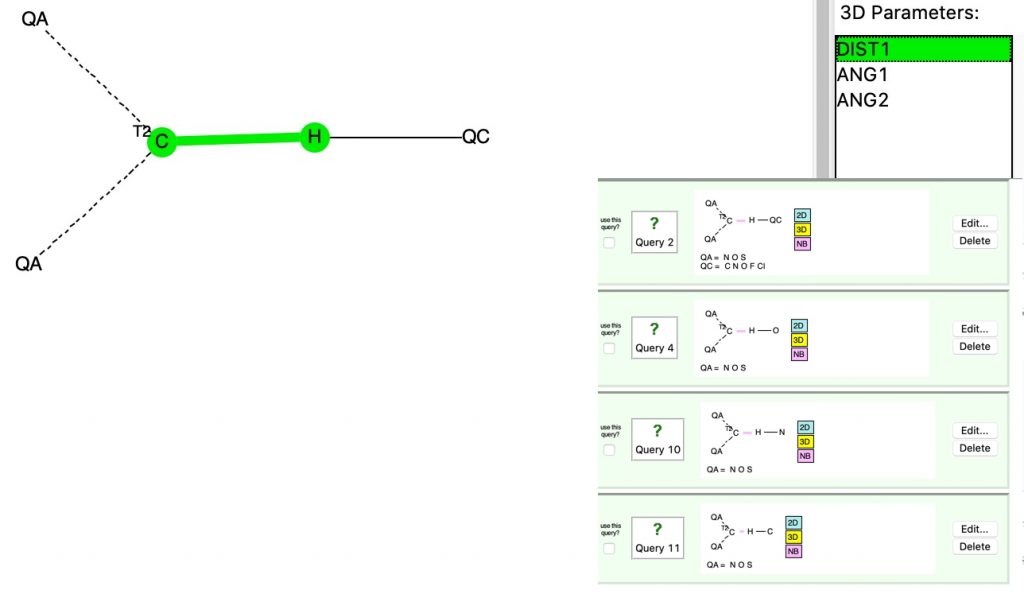
A hydrogen bond donor is considered as an electronegative element carrying a hydrogen that is accepted by an atom carrying a lone pair of electrons, as in X:…H-Y where X: is the acceptor and H-Y the donor. Wikipedia asserts that carbon can act as a donor, as we saw in the post on the incredible chloride cage, where six Cl**:**…H-C interactions trapped the chloride ion inside the cage.
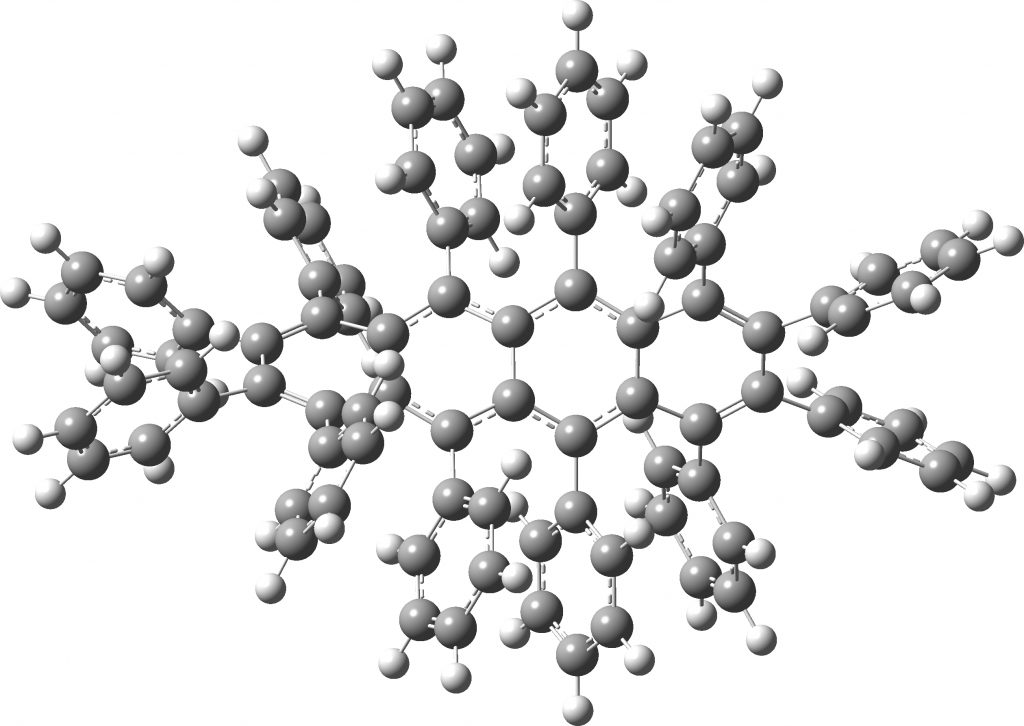
All of the molecules in this year’s C&EN list are fascinating in their very different ways. Here I take a look at the twisty tetracene (dodecaphenyltetracene) which is indeed very very twisty.[cite]10.1002/anie.201812418[/cite] Click on image to view 3D model Unfortunately, the authors point that the twisty-ness does not lead to a stable helical configuration at room temperatures and so separate enantiomers cannot be isolated.
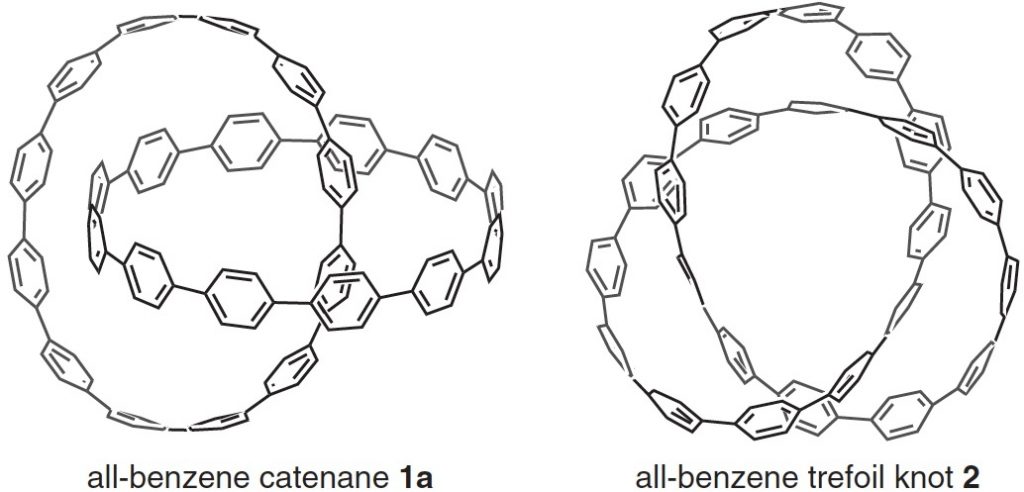
Here is another molecule of the year, on a topic close to my heart, the catenane systems 1 and the trefoil knot 2 [cite]10.1126/science.aav5021[/cite] Such topology is closely inter-twinned with three dimensions (literally) and I always find that the flat pages of a journal are simply insufficient to do them justice. So I set about finding the 3D coordinates.
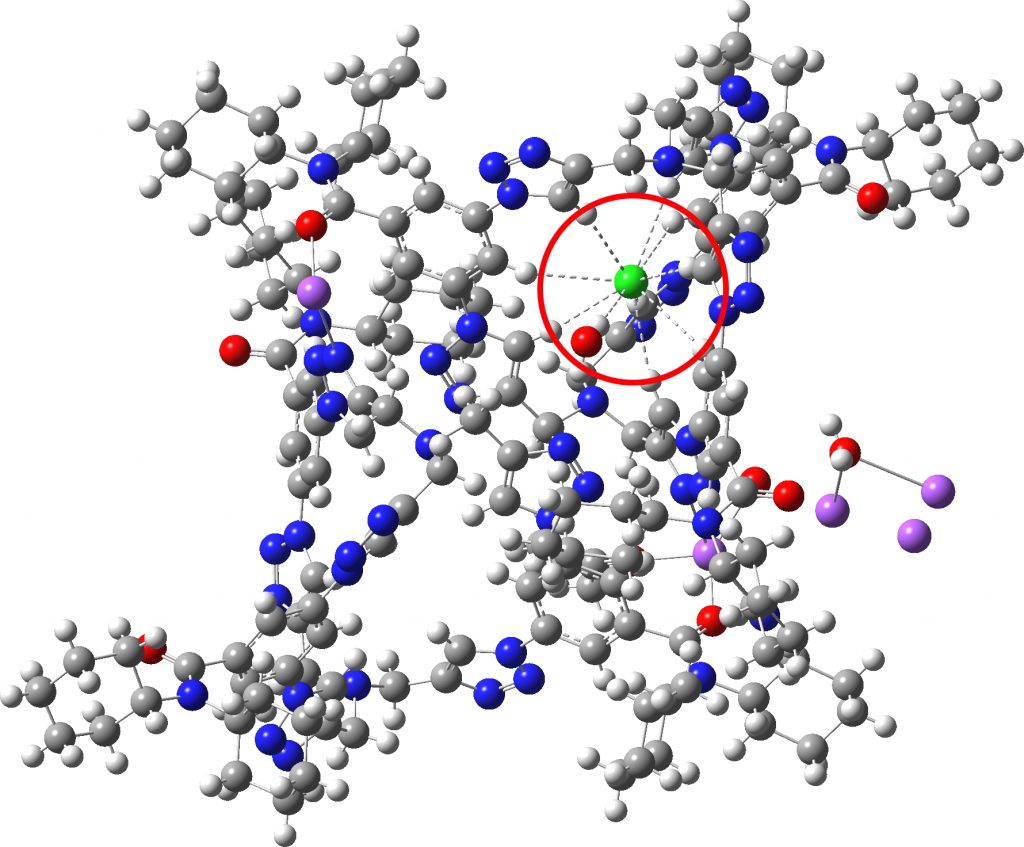
Each year, C&E News runs a poll for their “ Molecule of the year ”. I occasionally comment with some aspect of one of the molecules that catches my eye (I have already written about cyclo[18]carbon, another in the list). Here, it is the Incredible chloride cage , a cryptand-like container with an attomolar (10 17 M -1 ) affinity for a chloride anion.[cite]10.1126/science.aaw5145[/cite] The essence of
I have been discussing some historical aspects of the absolute configuration of molecules and how it was connected to their optical rotations. The nomenclature for certain types of molecules such as sugars and less commonly amino acids includes the notation (+) to indicate that the specific optical rotation of the molecule has a positive (rather than a negative) value.
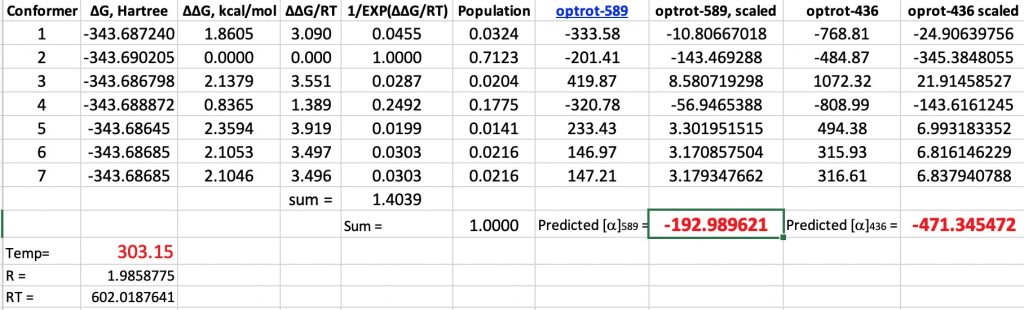
In this series of posts on optical rotations, I firstly noted Kirkwood’s 1937 attempt to correlate the optical rotation of butan-2-ol with its absolute configuration.