My previous post related to the aromatic electrophilic substitution of benzene using as electrophile phenyl diazonium chloride. Another prototypical reaction, and again one where benzene is too inactive for the reaction to occur easily, is the catalyst-free bromination of benzene to give bromobenzene and HBr.
Postagens de Rogue Scholar
The diazo-coupling reaction dates back to the 1850s (and a close association with Imperial College via the first professor of chemistry there, August von Hofmann) and its mechanism was much studied in the heyday of physical organic chemistry.[cite]10.1021/ja00830a009[/cite] Nick Greeves, purveyor of the excellent ChemTube3D site, contacted me about the transition state (I have commented previously on this aspect of aromatic
A staple of introductory undergraduate teaching in organic chemistry is Markovnikov’s rule, which states: “ the addition of a protic acid HX to an alkene results in the acid hydrogen (H) becoming attached to the carbon with fewer alkyl substituents and the halide (X) group to the carbon with more alkyl substituents ”. Shortly thereafter, students are exposed to the “anti-Markovnikov” addition of borane to e.g.
The potential energy surface for a molecule tells us about how it might react. These surfaces have been charted for thousands of reactions using quantum mechanics, and their basic features are thought to be well understood. Coming across an entirely new feature is rare. So what do you make of the following? The reaction is shown above[cite]10.1039/P19920001709 [/cite], and on the face of it, it looks like a normal pericyclic cascade.
A game one can play with pericyclic reactions is to ask students to identify what type a given example is. So take for example the reaction below. The alternatives are: A cyclo-elimination reaction (red arrows). Two concurrent electrocyclic ring openings (blue and magenta arrows) Two consecutive electrocyclic ring openings Or could it be a hybrid with characteristics of both the first two?
As my previous post hints, I am performing my annual spring-clean of lecture notes on pericyclic reactions. Such reactions, and their stereochemistry, are described by a set of selection rules . I am always on the lookout for a simple example which can most concisely summarise these rules. The (hypothetical) one shown below I think nicely achieves this, and raises some interesting issues in the process.
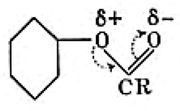
I have several times used arrow pushing on these blogs. But since the rules for this convention appear to be largely informal, and there appears to be no definitive statement of them, I thought I would try to produce this for our students. This effort is here shared on my blog. It is what I refer to as the standard version; an advanced version is in preparation. Such formality might come as a surprise to some;
Not long ago, I described a cyclic carbene in which elevating the carbene lone pair into a π-system transformed it from a formally 4n-antiaromatic π-cycle into a 4n+2 aromatic π-cycle. From an entirely different area of chemistry, another example of this behaviour emerges; Schreiner’s[cite]10.1039/C2SC21555A[/cite] trapping and reactions of t-butyl-hydroxycarbene, as described on Steve Bachrach’s blog.
The concept of kinetic vs thermodynamic control of a reaction is often taught in the context of the enolisation of e.g. 1-methylcyclohexanone as induced by a base.
The best known example of the gauche effect is 1,2-difluoroethane, which exhibits a relatively small preference of ~0.5 kcal/mol for this conformer over the anti orientation, which is also a minimum. But FSSF, which I discussed in the previous post, beats this hands down!