σ or π nucleophilic reactivity of imines? A mechanistic twist emerges.
The story so far. Imines react with a peracid to form either a nitrone (σ-nucleophile) or an oxaziridine (π-nucleophile).[1] The balance between the two is on an experimental knife-edge, being strongly influenced by substituents on the imine. Modelling these reactions using the “normal” mechanism for peracid oxidation did not reproduce this knife-edge, with ΔΔG (π-σ) 16.2 kcal/mol being rather too far from a fine balance.
There are two general reasons why computational modelling using quantum mechanics may not match experimental outcome. Until perhaps 10 or so years ago, the culprits may often have been the approximations necessary to apply the theory, as bounded by the limitations of the CPU power of the then available computers to evaluate the associated equations. Nowadays, an equally likely explanation is that the molecular model for which these equations are solved is either wrong or maybe just incomplete. For an organic reaction, these models are initially set out by “arrow pushing” a possible mechanistic pathway. Such speculations have been a common feature of most new articles reporting the outcome of reaction experiments for perhaps 60 years now. It is now more common (but by no means universal) to augment this with a computational reality check. So previously, when I applied a reality check on the “standard” epoxidation mechanism, it did not pass the test.
So time to revise the mechanism, as per below. The difference is that the model includes an extra water molecule to facilitate proton transfers, with the imine now being protonated by the peracid to form a zwitterion, which collapses to an addition product and it is this species that rearranges to the final oxaziridine. Free energies relative to the reactant 1 are shown in red below.‡
The IRC for 2 (TS) is shown below, being a proton transfer mediated by the transfer agent (water in this case, but it could be also peracid or eventually the product acid) to form a ion-pair.
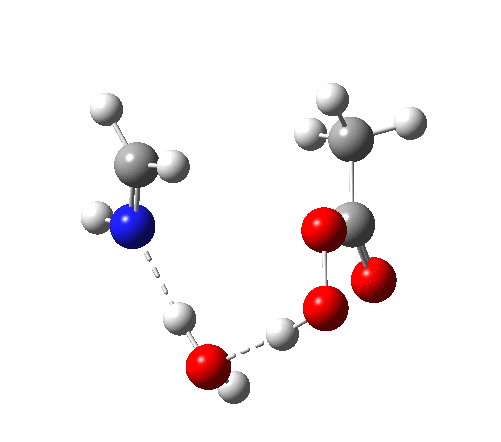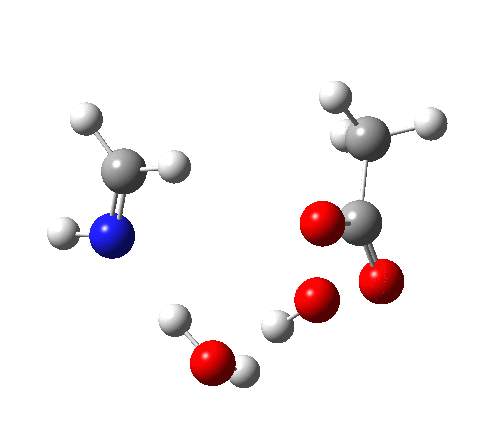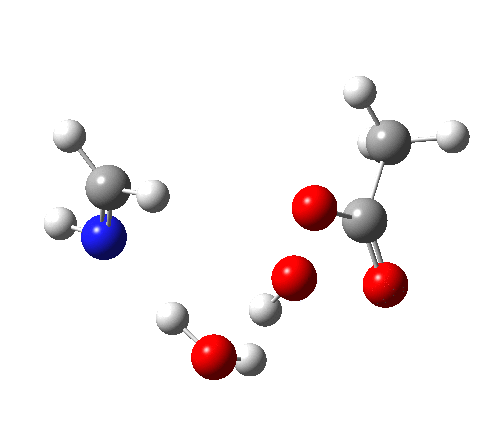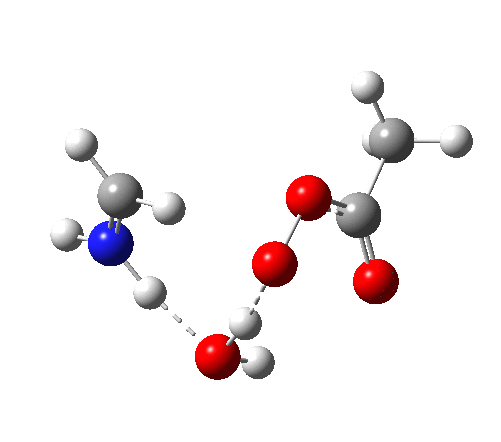
4 (TS) shows the collapse of the ion-pair to form an addition product across the imine.
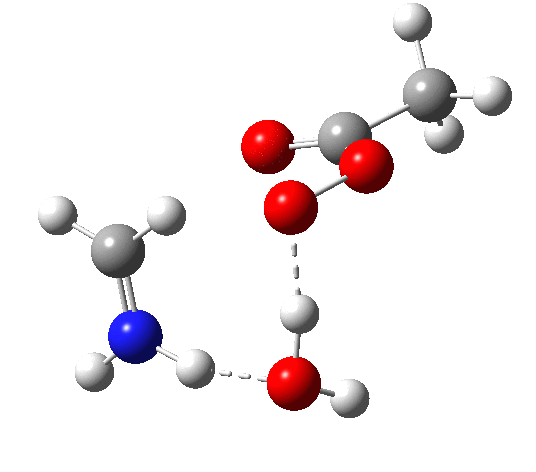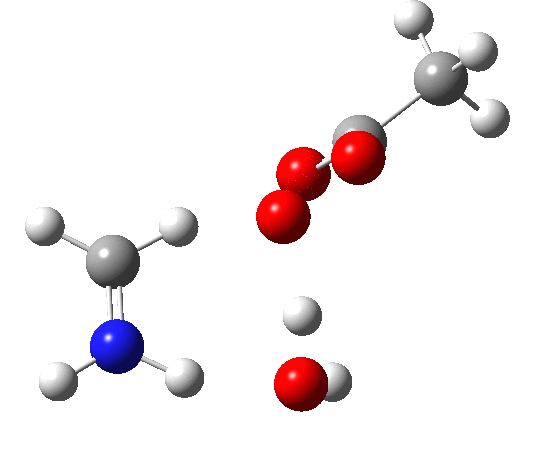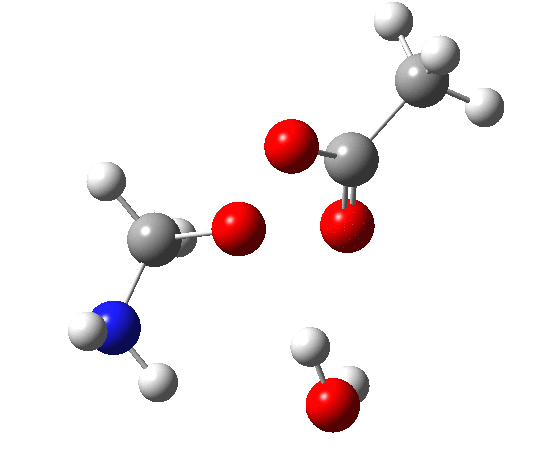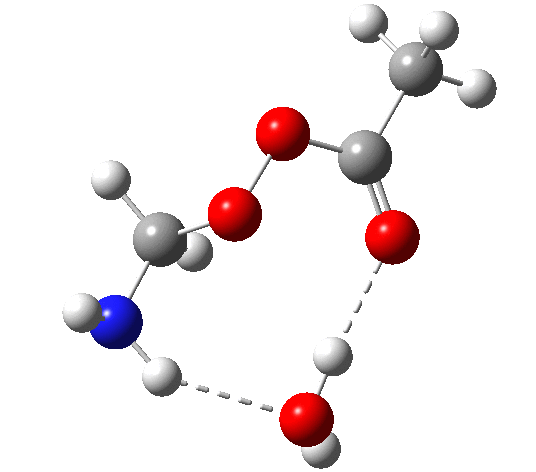
6 (TS, below) is the most interesting and also the high point on the free-energy pathway (i.e. the rate determining step). The addition product cyclises to an oxaziridine as induced by the nitrogen lone pair helping to evict the acetate anion. This is followed at IRC ~7 by a transfer of the N-H proton back to the carboxylic acid, again using water as a transfer agent with the whole being part of a concerted but asynchronous mechanistic step.
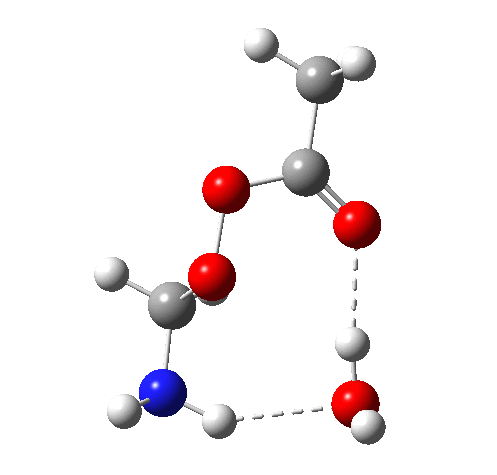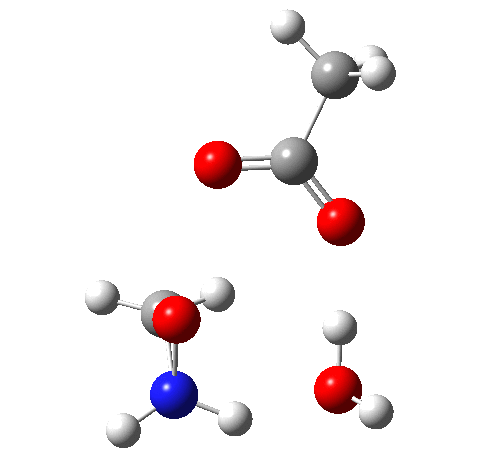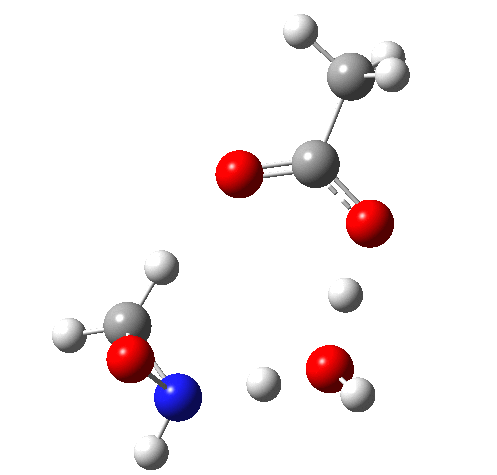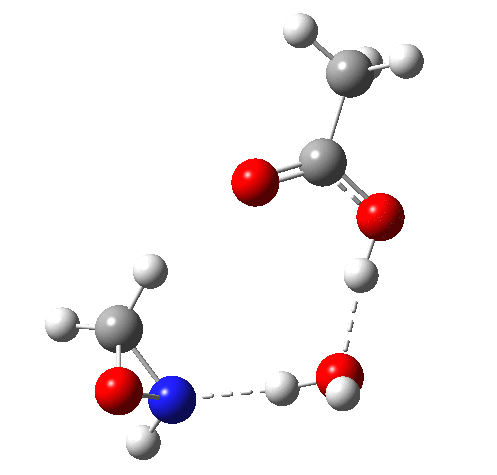
Crucially, 6 (TS) is 23.4 kcal/mol below the oxaziridination transition state modelled without a prior proton transfer[2],[3] and even 7.6 kcal/mol below the transition state for nitrone formation.[4],[5]
So the original mechanism is now replaced by an alternative, which really only differs in the timing of how the acidic proton attached to the peracid responds to the process. By getting actively involved prior to the crucial reaction with the nitrogen lone pair of the imine, this proton enables a lower energy route to be established. We are now ready for the next “reality check” on these mechanisms, which are the effects of substituents on the imine. If these can be replicated, we can then really start to claim that computation has put the mechanism of this reaction onto a firmer footing than that based just on “arrow-pushing”.
‡Calculations (ωB97XD/Def2-TZVPP/SCRF=dichloromethane) for the species above are archived as a collection at DOI: 10.14469/hpc/1704[6] and individually at 1[7], 2 (TS)[8], 3[9], 4 (TS)[10], [11], 5[12], 6 (TS)[13],[14], 7[15].
References
- D.R. Boyd, P.B. Coulter, N.D. Sharma, W. Jennings, and V.E. Wilson, "Normal, abnormal and pseudo-abnormal reaction pathways for the imine-peroxyacid reaction", Tetrahedron Letters, vol. 26, pp. 1673-1676, 1985. https://doi.org/10.1016/s0040-4039(00)98582-4
- H. Rzepa, "Imine + peracetic acid, π attack + H2O, TS.", 2016. https://doi.org/10.14469/hpc/1698
- H. Rzepa, "Imine + peracetic acid, pi attack + H2O, TS. IRC", 2016. https://doi.org/10.14469/hpc/1701
- H. Rzepa, "Imine + peracetic acid,N attack + H2O, TS", 2016. https://doi.org/10.14469/hpc/1697
- H. Rzepa, "Imine + peracetic acid,N attack + H2O, TS IRC", 2016. https://doi.org/10.14469/hpc/1702
- H. Rzepa, "Imine + peracetic acid, pi attack zwitterion + H2O reactant", 2016. https://doi.org/10.14469/hpc/1695
- H. Rzepa, "Imine + peracetic acid, π attack zwitterion + H2O", 2016. https://doi.org/10.14469/hpc/1703
- H. Rzepa, "Imine + peracetic acid, π attack zwitterion + H2O intermediate", 2016. https://doi.org/10.14469/hpc/1696
- H. Rzepa, "Imine + peracetic acid, pi attack zwitterion + H2O IRC => C-O formation TS", 2016. https://doi.org/10.14469/hpc/1692
- H. Rzepa, "Imine + peracetic acid, pi attack zwitterion + H2O IRC => C-O formation TS IRC", 2016. https://doi.org/10.14469/hpc/1700
- H. Rzepa, "Imine + peracetic acid, pi attack zwitterion + H2O TS IRC, addition int", 2016. https://doi.org/10.14469/hpc/1690
- H. Rzepa, "Imine + peracetic acid, pi attack zwitterion + H2O TS", 2016. https://doi.org/10.14469/hpc/1694
- H. Rzepa, "Imine + peracetic acid, π attack zwitterion + H2O TS IRC", 2016. https://doi.org/10.14469/hpc/1693
- H. Rzepa, "Imine + peracetic acid, pi attack zwitterion + H2O TS IRC, oxaziridine product", 2016. https://doi.org/10.14469/hpc/1691
Additional details
Description
The story so far. Imines react with a peracid to form either a nitrone (σ-nucleophile) or an oxaziridine (π-nucleophile). The balance between the two is on an experimental knife-edge, being strongly influenced by substituents on the imine.
Identifiers
- UUID
- d9e9507c-497a-4ddd-b672-f71ed83a593c
- GUID
- http://www.ch.imperial.ac.uk/rzepa/blog/?p=16902
- URL
- https://www.ch.ic.ac.uk/rzepa/blog/?p=16902
Dates
- Issued
-
2016-09-28T09:37:55
- Updated
-
2016-10-03T10:58:00
References
- Boyd, D. R., Coulter, P. B., Sharma, N. D., Jennings, W. B., & Wilson, V. E. (1985). Normal, abnormal and pseudo-abnormal reaction pathways for the imine-peroxyacid reaction. Tetrahedron Letters, 26(13), 1673–1676. https://doi.org/10.1016/s0040-4039(00)98582-4
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, π attack + H2O, TS. [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1698
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, pi attack + H2O, TS. IRC [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1701
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid,N attack + H2O, TS [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1697
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid,N attack + H2O, TS IRC [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1702
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, pi attack zwitterion + H2O reactant [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1695
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, π attack zwitterion + H2O [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1703
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, π attack zwitterion + H2O intermediate [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1696
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, pi attack zwitterion + H2O IRC => C-O formation TS [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1692
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, pi attack zwitterion + H2O IRC => C-O formation TS IRC [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1700
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, pi attack zwitterion + H2O TS IRC, addition int [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1690
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, pi attack zwitterion + H2O TS [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1694
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, π attack zwitterion + H2O TS IRC [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1693
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2016). Imine + peracetic acid, pi attack zwitterion + H2O TS IRC, oxaziridine product [Data set]. Imperial College London. https://doi.org/10.14469/hpc/1691
