5-Imino-5λ4-heptathiepane 3-oxide. More exuberent anomeric effects.
The two previous posts[1],[2] on the topic of anomeric effects in 7-membered sulfur rings illustrated how orbital interactions between the lone pairs in the molecules and S-S bonds produced widely varying S-S bond lengths in the molecules, some are shorter than normal (which is ~2.05Å for e.g. the S8 ring) by ~ 0.1Å and some are longer by ~0.24Å. Here we extend this to the unknown molecule shown below.
The usual MN15L/Def2-TZVPP calculation[3] gives the calculated geometry shown below. In parentheses are the calculated S-S vibrational wavenumbers (some are marked with ~ since these modes are contaminated by mixing with other parts of the molecules).
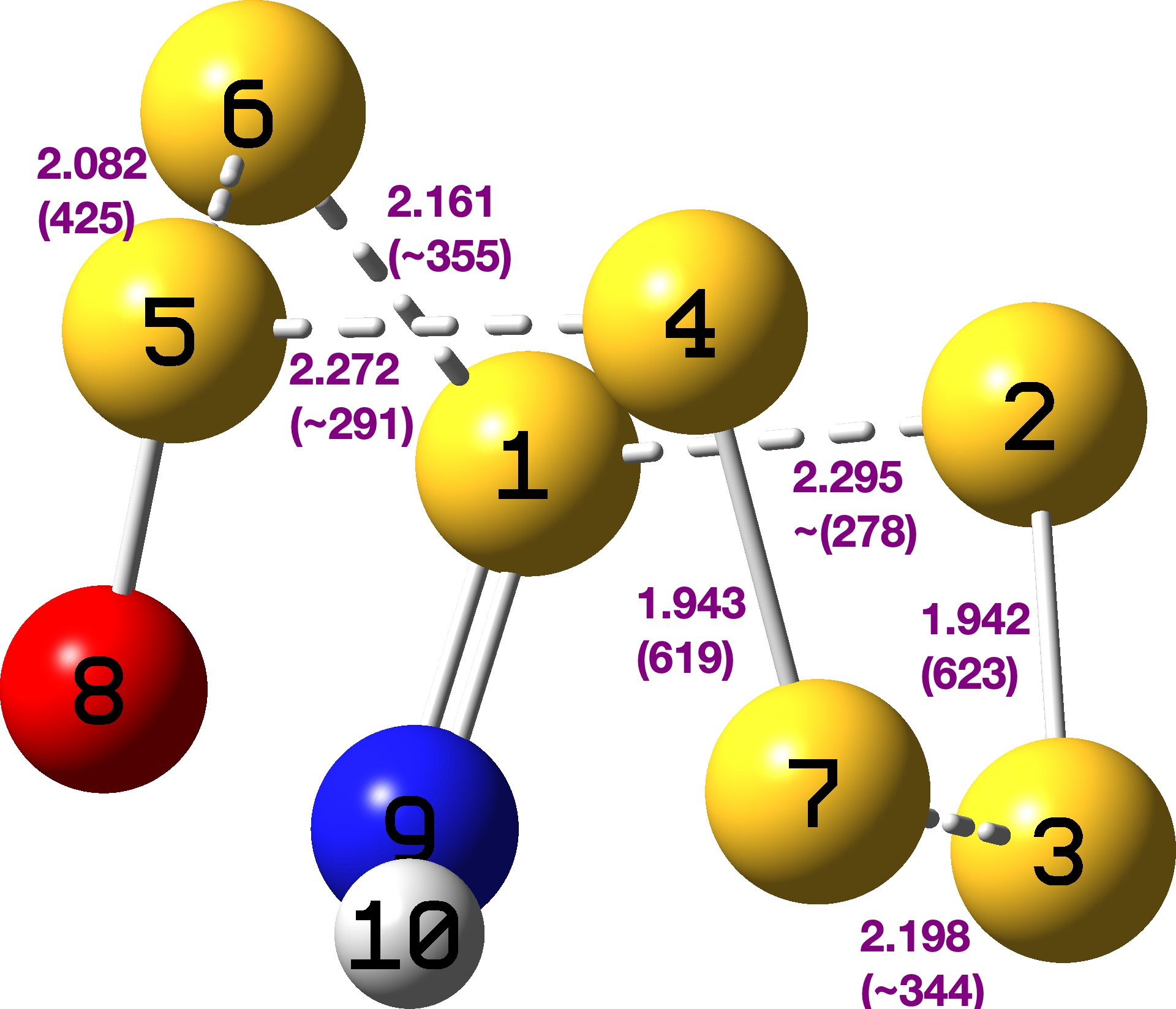
The interaction energies between the donor and acceptor, E(2), are shown below. Numbers 5-8 are the same as was identified for the parent molecule S7, but the energies have increased substantially (previously 12.3/10.1 kcal/mol). The Wiberg bond index for the strongest bond (S2-S3) is 1.276 and the weakest (S1-S2) is 0.610, quite some variation! Given that the known S7O was already very unstable[4], it seems unlikely that the probably even more unstable S7ONH could ever be isolated, but there is a challenge!
| # | Acceptor S-S bond | Donor Lp | NBO E(2) Energy |
|---|---|---|---|
| 1 | S4-S5 | O8 | 31.9 |
| 2 | S1-S2 | N9 | 29.6 |
| 3 | S1-S6 | N9 | 27.0 |
| 4 | S5-S6 | O8 | 20.4 |
| 5 | S4-S5 | S7 | 16.8 |
| 6 | S1-S2 | S3 | 16.4 |
| 7 | S3-S7 | S2 | 15.4 |
| 8 | S3-S7 | S4 | 15.2 |
There are numerous compounds with six, seven and eight membered sulfur rings, and it would always be worth keeping an eye out for unusually short or long S-S bonds in them, since they may well be more manifestations of these sulfur anomeric effects.
References
- H. Rzepa, "Cycloheptasulfur sulfoxide, S<sub>7</sub>O – Anomeric effects galore!", 2025. https://doi.org/10.59350/rzepa.28515
- H. Rzepa, "Cyclo-Heptasulfur, S<sub>7</sub> – a classic anomeric effect discovered during a pub lunch!", 2025. https://doi.org/10.59350/rzepa.28407
- H. Rzepa, "S7O2 NBO7 Cs symmetry", 2025. https://doi.org/10.14469/hpc/15235
- R. Steudel, R. Reinhardt, and T. Sandow, "Bond Interaction in Sulfur Rings: Crystal and Molecular Structure of <i>cyclo</i>‐Heptasulfur Oxide, S<sub>7</sub>O", Angewandte Chemie International Edition in English, vol. 16, pp. 716-716, 1977. https://doi.org/10.1002/anie.197707161
Additional details
Description
The two previous posts, on the topic of anomeric effects in 7-membered sulfur rings illustrated how orbital interactions between the lone pairs in the molecules and S-S bonds produced widely varying S-S bond lengths in the molecules, some are shorter than normal (which is ~2.05Å for e.g. the S8 ring) by ~ 0.1Å and some […]
Identifiers
- UUID
- 04a855ad-31db-4d0f-8dd3-8d724cb10372
- GUID
- https://www.ch.ic.ac.uk/rzepa/blog/?p=28615
- URL
- https://www.ch.ic.ac.uk/rzepa/blog/?p=28615
Dates
- Issued
-
2025-05-20T10:56:50
- Updated
-
2025-05-29T13:06:28
References
- Rzepa, H. (2025, May 19). Cycloheptasulfur sulfoxide, S7O – Anomeric effects galore!. Henry Rzepa's Blog. https://doi.org/10.59350/rzepa.28515
- Rzepa, H. (2025, May 16). Cyclo-Heptasulfur, S7 – a classic anomeric effect discovered during a pub lunch!. Henry Rzepa's Blog. https://doi.org/10.59350/rzepa.28407
- Rzepa, H., Imperial College London, Imperial College Research Computing Service, & Rzepa, H. (2025). S7O2 NBO7 Cs symmetry [Data set]. Imperial College London. https://doi.org/10.14469/hpc/15235
- Steudel, R., Reinhardt, R., & Sandow, T. (1977). Bond Interaction in Sulfur Rings: Crystal and Molecular Structure of cyclo‐Heptasulfur Oxide, S7O. Angewandte Chemie International Edition in English, 16(10), 716–716. https://doi.org/10.1002/anie.197707161
