To be cyclobutadiene, or not to be, that is the question? You decide.
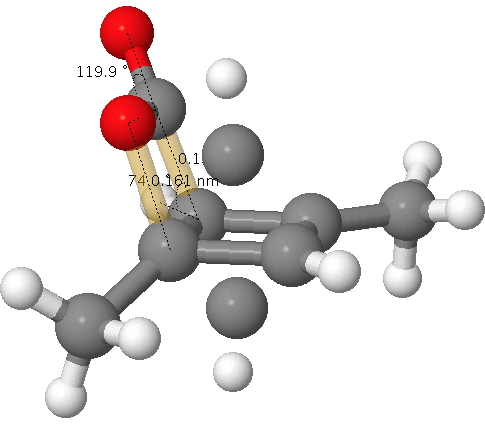
A quartet of articles has recently appeared on the topic of cyclobutadiene.[1],[2],[3],[4]. You will find a great deal discussed there, but I can boil it down to this essence. Do the following coordinates (obtained from a (disordered) previously published[5] x-ray refinement) correspond to a van der Waals complex of 1,3-dimethyl cyclobutadiene and carbon dioxide, or do they instead represent a covalent interaction between these two components resulting in a compound with the chemical name 2-oxabicyclo[2.2.0]hex-5-en-3-one (i.e. not a cyclobutadiene)?

Click for 3D. The unconnected atoms are the result of disordered partial occupancy.
The two bonds to concentrate on are shown in gold; a O…C pair with a distance of 1.61Å as obtained from the x-ray refinement and a C…C pair with a distance of 1.5Å (and if you want to go further, the O=C=O bond angle). I list below values obtained from the wonderful Webelements site. Using these values, this makes a van der Waals O…C contact 3.22Å and a C…C contact 3.40Å and covalent values of respectively 1.38Å and 1.5Å.
| Element | Covalent radius, Å | van der Waals radius |
| C | 0.75 | 1.70 |
| O | 0.63 | 1.52 |
According to chemistry convention, we classify the interaction between a pair of atoms according to which category best fits the observed distance. So this should allow you to decide if the molecule is a van der Waals complex of 1,3-dimethyl cyclobutadiene and carbon dioxide or the covalent system 2-oxabicyclo[2.2.0]hex-5-en-3-one.
Oh, if the observed O…C pair with a distance of 1.61Å does not seem to perfectly fit either category above, one of the quartet of articles above[1] offers the explanation of an unusual π-anomeric effect lengthening the C…O bond in 2-oxabicyclo[2.2.0]hex-5-en-3-one slightly beyond the standard covalent distance. Of course, if the system were to be a van der Waals complex, that explanation cannot apply.
References
- H.S. Rzepa, "A Computational Evaluation of the Evidence for the Synthesis of 1,3‐Dimethylcyclobutadiene in the Solid State and Aqueous Solution", Chemistry – A European Journal, vol. 19, pp. 4932-4937, 2013. https://doi.org/10.1002/chem.201102942
- M. Shatruk, and I.V. Alabugin, "Reinvestigation of "Single‐Crystal X‐ray Structure of 1,3‐dimethylcyclobutadiene"", Chemistry – A European Journal, vol. 19, pp. 4942-4945, 2013. https://doi.org/10.1002/chem.201103017
- Y. Legrand, D. Dumitrescu, A. Gilles, E. Petit, A. van der Lee, and M. Barboiu, "A Constrained Disorder Refinement: "Reinvestigation of "Single‐Crystal X‐ray Structure of 1,3‐Dimethylcyclobutadiene" by M. Shatruk and I. V. Alabugin"", Chemistry – A European Journal, vol. 19, pp. 4946-4950, 2013. https://doi.org/10.1002/chem.201203234
- Y. Legrand, D. Dumitrescu, A. Gilles, E. Petit, A. van der Lee, and M. Barboiu, "Reply to A Computational Evaluation of the Evidence for the Synthesis of 1,3‐Dimethylcyclobutadiene in Solid State and Aqueous Solution—Beyond the Experimental Reality", Chemistry – A European Journal, vol. 19, pp. 4938-4941, 2013. https://doi.org/10.1002/chem.201203235
- Y. Legrand, A. van der Lee, and M. Barboiu, "Single-Crystal X-ray Structure of 1,3-Dimethylcyclobutadiene by Confinement in a Crystalline Matrix", Science, vol. 329, pp. 299-302, 2010. https://doi.org/10.1126/science.1188002
Additional details
Description
A quartet of articles has recently appeared on the topic of cyclobutadiene.,,,. You will find a great deal discussed there, but I can boil it down to this essence.
Identifiers
- UUID
- 8c946f90-cdff-4246-b067-d59ca2694c4b
- GUID
- http://www.ch.imperial.ac.uk/rzepa/blog/?p=9894
- URL
- https://www.ch.ic.ac.uk/rzepa/blog/?p=9894
Dates
- Issued
-
2013-03-21T14:12:48
- Updated
-
2014-01-17T08:40:37
References
- Rzepa, H. S. (2013). A Computational Evaluation of the Evidence for the Synthesis of 1,3‐Dimethylcyclobutadiene in the Solid State and Aqueous Solution. Chemistry – A European Journal, 19(15), 4932–4937. https://doi.org/10.1002/chem.201102942
- Shatruk, M., & Alabugin, I. V. (2013). Reinvestigation of "Single‐Crystal X‐ray Structure of 1,3‐dimethylcyclobutadiene". Chemistry – A European Journal, 19(15), 4942–4945. https://doi.org/10.1002/chem.201103017
- Legrand, Y., Dumitrescu, D., Gilles, A., Petit, E., van der Lee, A., & Barboiu, M. (2013). A Constrained Disorder Refinement: "Reinvestigation of "Single‐Crystal X‐ray Structure of 1,3‐Dimethylcyclobutadiene" by M. Shatruk and I. V. Alabugin". Chemistry – A European Journal, 19(15), 4946–4950. https://doi.org/10.1002/chem.201203234
- Legrand, Y., Dumitrescu, D., Gilles, A., Petit, E., van der Lee, A., & Barboiu, M. (2013). Reply to A Computational Evaluation of the Evidence for the Synthesis of 1,3‐Dimethylcyclobutadiene in Solid State and Aqueous Solution—Beyond the Experimental Reality. Chemistry – A European Journal, 19(15), 4938–4941. https://doi.org/10.1002/chem.201203235
- Legrand, Y.-M., van der Lee, A., & Barboiu, M. (2010). Single-Crystal X-ray Structure of 1,3-Dimethylcyclobutadiene by Confinement in a Crystalline Matrix. Science, 329(5989), 299–302. https://doi.org/10.1126/science.1188002
